Abstract
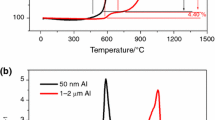
It is of great significance to study the thermal oxidation process to understand the reaction mechanism of aluminum particle and further its applications in propellants. The physical and chemical properties of micron-aluminum particle were evaluated by scanning electron microscopy, laser particle size analyzer, X-ray diffractometer and inductively coupled plasma atomic emission spectrometer. The thermal oxidation characteristics of the sample were studied by thermal analyzer. The experimental results showed that the initial oxide thickness of the sample was about 3.96 nm, and the calculated values of the specific surface area and the active aluminum content obtained by the established mathematical model were in good agreement with the measured values. The thermal oxidation process of the sample was divided into three stages. When the temperature rose to 1100 °C, the thermal oxidation efficiency of the sample reached 98.55%. With the increase in treatment temperature, dramatic crystalline changes occurred on the surface of the sample: amorphous alumina—γ-Al2O3, α-Al2O3, and the oxide layer thickness increased from 3.96 to 5.72 nm and 31.56 nm up to 320.15 nm. When the temperature reached 700 °C, the outer surface of the oxide layer contained a small amount of α-Al2O3, while the interior consisted of a large amount of γ-Al2O3, indicating that the conversion of γ-Al2O3 to α-Al2O3 occurred from the inside out.











Similar content being viewed by others
References
Badiola C, Gill RJ, Dreizin EL. Combustion characteristics of micron-sized aluminum particles in oxygenated environments. Combust Flame. 2011;158(10):2064–70.
Cheng H, Chen X, Zhou C, et al. Research progress on combustion characteristics of aluminum particles. J Ordnance Equip Eng. 2010;31(3):84–8.
Bazyn T, Krier H, Glumac N. Evidence for the transition from the diffusion-limit in aluminum particle combustion. Proc Combust Inst. 2007;31(2):2021–8.
Pang WQ, Fan XZ. Application progress of metal fuels in solid propellants. Chem Propellants Polym Mater. 2009;7(2):1–14.
Gill RJ, Badiola C, Dreizin EL. Combustion times and emission profiles of micron-sized aluminum particles burning in different environments. Combust Flame. 2010;157(11):2015–23.
Mohan S, Furet L, Dreizin EL. Aluminum particle ignition in different oxidizing environments. Combust Flame. 2010;157(7):1356–63.
Trunov MA, Schoenitz M, Dreizin EL. Effect of polymorphic phase transformations in alumina layer on ignition of aluminium particles. Combust Theor Model. 2006;10(4):603–23.
Zhang S, Dreizin EL. Reaction interface for heterogeneous oxidation of aluminum powders. J Phys Chem C. 2013;117(27):14025–31.
Rai A, Park K, Zhou L, et al. Understanding the mechanism of aluminium nanoparticle oxidation. Combust Theor Model. 2006;10(5):843–59.
Levitas VI, Pantoya ML, Dikici B. Melt dispersion versus diffusive oxidation mechanism for aluminum nanoparticles: critical experiments and controlling parameters. Appl Phys Lett. 2008;92(1):11921.
Trunov MA, Schoenitz M, Zhu X, et al. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust Flame. 2005;140(4):310–8.
Sun Y, Sun R, Zhu B, et al. Thermal reaction mechanisms of nano- and micro-scale aluminum powders in carbon dioxide at low heating rate. J Therm Anal Calorim. 2016;124(3):1727–34.
Rufino B, Boulc HF, Coulet MV, et al. Influence of particles size on thermal properties of aluminium powder. Acta Mater. 2007;55(8):2815–27.
Levin I, Brandon D. Metastable alumina polymorphs: crystal structures and transition sequences. J Am Ceram Soc. 1998;81(8):1995–2012.
Jeurgens LPH, Sloof WG, Tichelaar FD, et al. Thermodynamic stability of amorphous oxide films on metals: application to aluminum oxide films on aluminum substrates. Phys Rev B. 2000;62(7):4707–19.
Rouquerol F, Rouquerol J, Sing K. Adsorption by powders and porous solids. London: Academic Press; 1999.
Zhou H, Zhang YW, Li HP, et al. Study on thermal oxidation characteristics and kinetics of boron-based fuel-rich in different atmosphere. J Zhejiang Univ. 2013;47(11):1987–91.
Hu Z, Shi Q. Thermal analysis kinetics. Beijing: Bejing Science Press; 2001.
Brooks KP, Beckstead MW. Dynamics of aluminum combustion. J Propul Power. 1995;11(4):769–80.
Yunan Z, Jianzhong L, Jiahao W, et al. Progress in oxidation mechanism and combustion theory of aluminum particles. Ordnance Mater Sci Eng. 2017;40(2):28–35.
Tian R, Zhang L. Literature review of single aluminum combustion model and theoretical research. J Ordnance Equip Eng. 2016;7:137–43.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 51376160) and the National Natural Science Foundation of China (No. 51706057).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Liu, J., Wang, J. et al. The formation mechanism and distribution of micro-aluminum oxide layer. J Therm Anal Calorim 133, 1335–1344 (2018). https://doi.org/10.1007/s10973-018-7174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7174-2