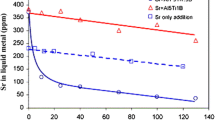
Abstract
Thermal and microscopy analyses were carried out to investigate the interaction of Sr modification with Ca and P trace elements in high-purity and commercial-purity Al–5Si–1Cu–Mg alloys. The results show how the addition of Sr to commercial-purity alloy induces significant changes in the nucleation and growth temperatures of eutectic Si since pre-eutectic Al2Si2(CaSr) intermetallics tend to poison AlP particles, making them inactive as nucleation sites for eutectic Si. In contrast, the addition of Sr to high-purity alloy shows no apparent influence on the characteristic temperatures of Al–Si eutectic reaction, even though the microstructural investigations reveal flake-to-fibrous transition in the eutectic Si structure. This indicates that the eutectic growth temperature, commonly used to predict eutectic modification level, is not a key feature of Sr modification, but it is indirectly caused due to the presence of additional impurities in commercial-purity alloys which affect the nucleation kinetics of eutectic Si.














Similar content being viewed by others
References
Ludwig TH. Trace elements in Al–Si foundry alloys. Trondheim: University of Science and Technology (NTNU); 2013.
Tański T, Labisz K, Krupińska B, Krupiński M, Król M, Maniara R, Borek W. Analysis of crystallization kinetics of cast aluminum–silicon alloy. J Therm Anal Calorim. 2016;123:63–74.
Mahfoud M, Prasada Rao AK, Emadi D. The role of thermal analysis in detecting impurity levels during aluminum recycling. J Therm Anal Calorim. 2010;100:847–51.
EN 1706:2010. Aluminium and aluminium alloys—castings—chemical composition and mechanical properties.
Gruzleski J, Closset B. The treatment of liquid aluminum–silicon alloys. Schaumburg: American Foundrymen’s Society; 1990.
Knuutinen A, Nogita K, McDonald SD, Dahle AK. Modification of Al–Si alloys with Ba, Ca, Y and Yb. J Light Met. 2001;1:229–40.
Kobayashi T, Kim HJ, Niinomi M. Effect of calcium on mechanical properties of recycled aluminium casting alloys. Mater Sci Technol. 1997;13:497–502.
Nogita K, Knuutinen A, McDonald SD, Dahle AK. Mechanisms of eutectic solidification in Al–Si alloys modified with Ba, Ca, Y and Yb. J Light Met. 2001;1:219–28.
Ludwig TH, Schaffer PL, Arnberg L. Influence of some trace elements on solidification path and microstructure of Al–Si foundry alloys. Metall Mater Trans A. 2013;44:3783–96.
Knuutinen A, Nogita K, McDonald SD, Dahle AK. Porosity in aluminium alloy A356 modified with Ba, Ca, Y and Yb. J Light Met. 2001;1:241–9.
Dai HS, Liu XF. Optimal holding temperatures and phosphorus additions for primary silicon refinement in Al—high Si alloys. Mater Sci Technol. 2009;25:1183–8.
Ludwig TH, Schaffer PL, Arnberg L. Influence of phosphorus on the nucleation of eutectic silicon in Al–Si alloys. Metall Mater Trans A. 2013;44:5796–805.
Al-Helal K, Wang Y, Stone I, Fan Z. Effect of Ca level on the formation of silicon phases during solidification of hypereutectic Al–Si alloys. Mater Sci Forum. 2013;765:117–22.
Qiao J, Liu X, Liu X, Bian X. Relationship between microstructures and contents of Ca/P in near-eutectic Al–Si piston alloys. Mater Lett. 2005;59:1790–4.
Ludwig TH, Schonhovd Dæhlen E, Schaffer PL, Arnberg L. The effect of Ca and P interaction on the Al–Si eutectic in a hypoeutectic Al–Si alloy. J Alloys Compd. 2014;586:180–90.
Farahany S, Idris MH, Ourdjini A, Faris F, Ghandvar H. Evaluation of the effect of grain refiners on the solidification characteristics of an Sr-modified ADC12 die-casting alloy by cooling curve thermal analysis. J Therm Anal Calorim. 2015;119:1593–601.
Farahany S, Ourdjini A, Idris MH, Shabestari SG. Computer-aided cooling curve thermal analysis of near eutectic Al–Si–Cu–Fe alloy: effect of silicon modifier/refiner and solidification conditions on the nucleation and growth of dendrites. J Therm Anal Calorim. 2013;114:705–17.
Farahany S, Ourdjini A, Idris MH. The usage of computer-aided cooling curve thermal analysis to optimise eutectic refiner and modifier in Al–Si alloys. J Therm Anal Calorim. 2012;109:105–11.
Samuel A, Doty H, Gallardo S, Samuel F. The effect of Bi–Sr and Ca–Sr interactions on the microstructure and tensile properties of Al–Si-based alloys. Mater. 2016;9:1–13.
Mrówka-Nowotnik G, Sieniawski J. Microstructure and mechanical properties of C355.0 cast aluminium alloy. Arch Mater Sci Eng. 2011;47:85–94.
Djurdjevic MB, Vicario I, Huber G. Review of thermal analysis applications in aluminium casting plants. Rev Metal. 2014;50:1–12.
Tamminen J. Thermal analysis for investigation of solidification mechanisms in metals and alloys. Stockholm: University of Stockholm; 1988.
Dahle AK, Nogita K, McDonald SD, Dinnis C, Lu L. Eutectic modification and microstructure development in Al–Si Alloys. Mater Sci Eng A. 2005;413:243–8.
Dahle AK, Nogita K, Zindel JW, McDonald SD, Hogan LM. Eutectic nucleation and growth in hypoeutectic Al–Si alloys at different strontium levels. Metall Mater Trans A. 2001;32:949–60.
McDonald SD, Nogita K, Dahle AK. Eutectic nucleation in Al–Si alloys. Acta Mater. 2004;52:4273–80.
Eiken J, Apel M, Liang S-M, Schmid-Fetzer R. Impact of P and Sr on solidification sequence and morphology of hypoeutectic Al–Si alloys: combined thermodynamic computation and phase-field simulation. Acta Mater. 2015;98:152–63.
Cho YH, Lee HC, Oh KH, Dahle AK. Effect of strontium and phosphorus on eutectic Al–Si nucleation and formation of β-Al5FeSi in hypoeutectic Al–Si foundry alloys. Metall Mater Trans A. 2008;39:2435–48.
Zamani M, Seifeddine S. Assessment of modification level in EN AC-46000 aluminum casting alloys using thermal analysis and microscopic evaluation. In: Hyland M, editor. Light metals 2015. Cham: Springer; 2015. p. 955–60.
Malekan M, Dayani D, Mir A. Thermal analysis study on the simultaneous grain refinement and modification of 380.3 aluminum alloy. J Therm Anal Calorim. 2014;115:393–9.
Malekan M, Shabestari SG. Computer-aided cooling curve thermal analysis used to predict the quality of aluminum alloys. J Therm Anal Calorim. 2011;103:453–8.
Rakhmonov J, Timelli G, Bonollo F. Influence of melt superheat, Sr modifier, and Al–5Ti–1B grain refiner on microstructural evolution of secondary Al–Si–Cu alloys. Metall Mater Trans A. 2016;47:5510–21.
Acknowledgements
The authors would like to acknowledge Dr. A. Fabrizi for his precious work to perform experiments and characterizations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakhmonov, J., Timelli, G. & Basso, G. Interaction of Ca, P trace elements and Sr modification in AlSi5Cu1Mg alloys. J Therm Anal Calorim 133, 123–133 (2018). https://doi.org/10.1007/s10973-018-7111-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7111-4