Abstract
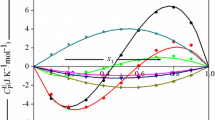
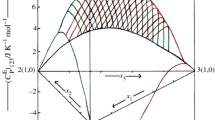
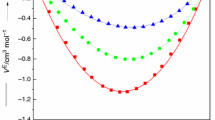
In the present study, molar heat capacities, \(\left( {C_{\text{P}}^{{}} } \right)_{123}\), at T/K = 293.15–308.15 K with 5 K interval and excess molar enthalpies, \(H_{ 1 2 3}^{\text{E}}\), at T/K = 308.15 for ternary tetrahydropyran (1) + piperidine (2) + cyclohexanone or cycloheptanone (3) and molar heat capacities, \((C_{\text{P}}^{{}} )_{12}\), for binary tetrahydropyran (1) + piperidine (2) mixture at T/K = 293.15, 298.15, 303.15 and 308.15 have been determined as a function of composition using microdifferential scanning calorimeter. The \(\left( {C_{\text{P}}^{\text{E}} } \right)_{123}\), \(H_{123}^{\text{E}}\) and \((C_{\text{P}}^{\text{E}} )_{12}\) data have been fitted to Redlich–Kister equation to predict ternary and binary adjustable parameters along with standard deviation in the measured properties. The observed \(\left( {C_{\text{P}}^{\text{E}} } \right)_{123}\), \(H_{123}^{\text{E}}\) data have been tested in terms of graph theory which in turns deals with the topology of the constituent molecules. It has been observed that \(\left( {C_{\text{P}}^{\text{E}} } \right)_{123}\) and \(H_{123}^{\text{E}}\) values estimated by graph theory are comparable with the experimental data.




Similar content being viewed by others
References
Selvi CS, Valliammai CT, Malliga P, Thenmozhi C, Kannappan V. Ultrsonic and molecular interaction studies of cinnamaldehyde with acetone in n-hexane. Rasayan J Chem. 2017;10:271–8.
Shihab SS, Rao KG, Kiran MG, Babu S, Sastry SS. Excess thermodynamic and acoustic properties for equimolar mixture of methyl benzoate and alkanols with benzene at 303.15 K. Rasayan J Chem. 2017;10:59–63.
Zarei H, Omidi A. Experimental study on the calorimetric data of 2-butoxyethanol with the Wilson, NRTL and UNIQUAC models at 298.15 K. J Chem Thermodyn. 2016;103:30–5.
Zarei H, Bohloor F, Omidi A. Excess molar enthalpies of ethane-1,2-diamine plus primary and secondary alkanols (C1-C4) and correlation with Redlich-Kister, Wilson, NRTL and UNIQUAC models at T = 298 K. J Chem Thermodyn. 2017;107:163–9.
Xie X, Zhao T, Guo B, Sha F, Qiao X, Zhang J. Density, viscosity and excess properties for the binary system 2-butoxy ethanol + water at T = (298.15–318.15) K and mixture’s spectroscopic studies. Phys Chem Liq. 2017;55:589–604.
Iloukhani H, Soleimani M, Khanlarzadeh K. The study of physico-chemical properties of binary systems consisting of N-Methylcyclohexylamine with 2-alkanols at T = (298.15 to 328.15) K. J Chem Thermodyn. 2017;110:110–26.
Sim HW, Kim MG. Excess volume and excess enthalpy of binary mixtures composed of 1,2-dichloropropane and 1-alkanol (C5-C8) Korean. J Chem Eng. 2016;33:271–6.
Liu C, Ma L, Sha F, Qiao X, Zhang J. Experimental investigation of density, viscosity and intermolecular interaction of binary system 1,3-butanediol + 1,2-ethanediamine for CO2 capture. J Mol Liq. 2017;232:130–8.
Sekhar MC, Venkatesulu A, Gowrisankar M, Krishna TS. Thermodynamic study of interactions in binary liquid mixtures of 2-Chloroaniline with some carboxylic acids. Phys Chem Liq. 2017;55:196–217.
Chang CW, Hsiung TL, Lui CP, Tu CH. Densities, surface tensions, and isobaric vapor-liquid equilibria for the mixtures of 2-propanol, water, and 1,2-propanediol. Fluid Phase Equilib. 2015;389:28–40.
Bandres I, Royo FM, Gascon I, Castro M, Lafuente C. Anion influence on thermophysical properties of ionic liquids: 1- butylpyridinium tetrafluoroborate and 1-butylpyridinium triflate. J Phys Chem B. 2010;114:3601–7.
Zhang ZH, Tan ZC, Sun LX, Zhen YJ, Lv XC, Shi Q. Thermodynamic investigation of room temperature ionic liquid: the heat capacity and standard enthalpy of formation of EMIES. Thermochim Acta. 2006;447:141–6.
Sattari M, Gharagheizi F, Kashkouli PI, Mohammadi AH, Ramjugernath D. Development of a group contribution method for the estimation of heat capacities of ionic liquids. J Therm Anal Calorim. 2014;115:1863–82.
Checoni RE, Volpe PO. Measurements of the molar heat capacities and excess molar heat capacities for water + organic solvents mixture at 288.15 K to 303.15 K and atmospheric pressure. J Solut Chem. 2010;39:259–76.
Zorebski E, Chorazewski M, Tkaczyk M. Excess molar heat capacities for (1-butanol + 1,3-butanediol) at temperatures from (285 to 353) K. J Chem Thermodyn. 2005;37:281–7.
Letyanina I, Tsvetov N, Toikka A. Excess molar enthalpies of the ternary mixture n-propanol + acetic acid + water at 313.15 K and atmospheric pressure. Fluid Phase Equilib. 2015;405:150–6.
Batov DV, Kustov AV, Antonova OA, Smirnova NL. Thermal and volumetric properties of methanol-hexamethylphosphortriamide mixtures under standard conditions. Russ J Phys Chem A. 2017;91:323–9.
Abbas R, Gmehling J. Vapour–liquid equilibria, azeotropic data, excess enthalpies, activity coefficients at infinite dilution and solid–liquid equilibria for binary alcohol-ketone systems. Fluid Phase Equilib. 2008;267:119–26.
Zhang R, Chen J, Mi J. Excess molar enthalpies for binary mixtures of different amines with water. J Chem Thermodyn. 2015;89:16–21.
Poozesh S, Rayer AV, Henni A. Molar excess enthalpy (H Em ) for systems of aqueous piperazine derivatives. J Chem Thermodyn. 2015;90:242–50.
Khanlarzadeh K, Iloukhani H, Soleimani M. Investigation on molecular interactions of binary mixtures of isobutanol with 1-alkanols (C1–C6) at different temperatures. Application of the Peng–Robinson–Stryjek–Vera (PSRV) equation of state (EOS). J Mol Struct. 2017;1139:78–86.
Kiaee H, Rostami AA, Farmanzadeh D. Excess properties and spectroscopic studies for a binary system of polyethylene glycol and N-methyl-2-pyrrolidone at different temperatures. J Mol Liq. 2017;231:242–8.
Aparcio S, Alcalde R, Davila MJ, Garcia B, Leal JM. Measurement and predictive models for the N-methyl-2-pyrrolidone/water/methanol systems. J Phys Chem B. 2008;112:11361–73.
Lin R, Sun H, Yang C, Shen W, Xia W. Visible light-induced difunctionalization of electron-enriched styrenes: synthesis of tetrahydrofurans and tetrahydropyrans. Chem Commun. 2015;51:399–401.
Xianming W, Yasukawa E, Kasuya S. Electrochemical properties of tetrahydropyran-based ternary electrolytes for 4 V Lithium metal rechargeable batteries. Electrochim Acta. 2001;46:813–9.
Valles C, Perez E, Cardoso M, Dominguez M, Mainar AM. Excess enthalpy, density, viscosity and speed of sound for the mixture tetrahydropyran + 1-butanol at (283.15, 298.15, and 313.15) K. J Chem Eng Data. 2004;49:1460–4.
Gonzalez JA. Thermodynamics of mixtures containing amines. X. Systems with cyclic amines or morpholine. Ind Eng Chem Res. 2011;50:9810–20.
Gomez-Diaz D, Navaza JM. Surface behavior of aqueous solutions of pyrrolidine and piperidine. J Chem Eng Data. 2004;49:1406–9.
Bernauer M, Dohnal V. Temperature dependences of limiting activity coefficients with Henry’s law constants for N-methylpyrrolidone, pyridine, and piperidine in water. Fluid Phase Equilib. 2009;282:100–7.
Malik S, Chandrasekhar M, Krishna TS, Sharma VK. Thermodynamic properties of piperidine and cyclic alkanone mixtures: excess molar volumes, excess isentropic compressibilities, excess molar enthalpies and excess heat capacities. J Therm Anal Calorim. 2017;129:1751–65.
Sharma VK, Malik S, Solanki S. Thermodynamic studies of molecular interactions in mixtures containing tetrahydropyran, 1,4-dioxane, and cyclic ketones. J Chem Eng Data. 2017;62:623–32.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and method of purification. 4th ed. New York: Wiley Interscience; 1986.
Comelli F, Francesconi R, Bigi A, Rubini K. Molar heat capacities, densities, viscosities, and refractive indices of dimethylsulfoxide + tetrahydropyran and + 2-methyltetrahydrofuran at (293.15, 303.15, and 313.15) K. J Chem Eng Data. 2007;52:639–44.
Giner B, Oliver B, Giner I, Pera G, Lafuente C. Isentropic and excess isentropic compressibilities of binary mixtures containing cyclic ethers and chloroalkanes. J Solut Chem. 2007;36:375–86.
Nikolic A, Jovic B, Kristic V, Trickovic J. Excess molar volumes of N-methylformamide + tetrahydropyran, + 2-pentanone, + propylacetate at the temperatures between 298.15 and 318.15 K. J Mol Liq. 2007;133:39–42.
Rodriguez S, Lafuente C, Artigas CH, Royo FM, Urieta JS. Densities, speeds of sound, and isentropic compressibilities of a cyclic ether with chlorocyclohexane, or bromocyclohexane at the temperatures 298.15 and 313.15 K. J Chem Thermodyn. 1999;31:139–49.
Inglese A, Grolier JPE, Wilhelm E. Excess volumes and excess heat capacities of oxane + cyclohexane and 1,4-dioxane + cyclohexane. Fluid Phase Equilib. 1984;15:287–94.
Bravo R, Pintos M, Amigo A. Dependence upon temperature of the excess molar volumes of tetrahydropyran, + n-alkane mixtures. Can J Chem. 1995;73:375–9.
Boussebissi A, Belaribi GB, Belaribi FB. Volumetric properties of piperidine + 1-alkanol binary liquid mixtures experimental results and application of Prigogine–Flory–Patterson theory. J Mol Liq. 2014;196:1–6.
Diaz DG, Navaza JM. Speed of sound and isentropic compressibility of aqueous solutions of pyrrolidine and piperidine. J Chem Eng Data. 2006;51:722–4.
Afzal W, Valtz A, Coquelet C, Richon D. Volumetric properties of (piperidine + water) binary system: measurements and modeling. J Chem Thermodyn. 2008;40:47–53.
Dey Sanguri V. Theoretical estimations of thermodynamic properties of liquid mixtures by Flory’s statistical theory. Phys Chem Liq. 2008;46:417–32.
Hughes EAM, Thorpe PL. The physical and thermodynamic properties of some associated solutions. II. Heat capacities and compressibilities. Proc R Soc Lond Ser A Math Phys Sci. 1964;278:574–87.
Messerly JF, Todd SS, Finke HL, Good WD, Gammon BE. Condensed-phase heat-capacity studies and derived thermodynamic properties for six cyclic nitrogen compounds. J Chem Thermodyn. 1988;20:209–24.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3- methylimidazolium and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Palaiologou MM, Arianas GK, Tsierkezos NG. Thermodynamic investigation of dimethyl sulfoxide binary mixtures at 293.15 and 313.15 K. J Solut Chem. 2006;35:551–65.
Lange NA. Handbook of chemistry. 11th ed. New York: Mc Graw-Hill; 1973.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes for binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Rafiee HR, Ranjbar S, Poursalman F. Densities and viscosities of binary and ternary mixtures of cyclohexanone, 1,4-dioxane and isooctane from T = (288.15–313.15) K. J Chem Thermodyn. 2012;54:266–71.
Tsierkezos NG, Molinou IE, Filippou AC. Thermodynamic properties of binary mixtures of cyclohexanone with n-alkanols (C1–C5) at 293.15 K. J Solut Chem. 2005;34:1371–86.
Salguero CB, Fadrique JG, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Nishikawa K, Ohomura K, Tamura K, Murakami S. Excess thermodynamic properties of mixtures of cyclohexanone and benzene at 298.15 and 308.15 K and the effect of excess expansion factor. Thermochim Acta. 1995;267:323–32.
Sharma VK, Kataria J, Solanki S. Molecular interactions in binary mixtures of lactams with cyclic alkanones. J Solut Chem. 2014;43:486–524.
Sharma VK, Kataria J. Topological investigations of excess heat capacities of binary liquid mixtures containing lactams and cycloalkanones. J Mol Liq. 2013;188:210–21.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Sharma VK, Rohilla A. Excess heat capacities of 1-methylpyrrolindin-2-one and pyridine or picolines mixtures. Thermochim Acta. 2013;568:140–7.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium-tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sabbah R, An XW, Chickos JS, Leita MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Belaribi FB, Boukais-Belaribi G, Mohammadi AH, Richon D. Excess molar enthalpies for the binary and ternary mixtures of cyclohexane, tetrahydropyran, and piperidine at 308.15 K and atmospheric pressure: experimental measurements and correlations. J Chem Eng Data. 2010;55:303–7.
Singh PP, Bhatia M. Energetic of molecular interactions in binary mixtures of non-electrolytes containing a salt. J Chem Soc, Faraday Trans. 1989;85:3807–12.
Huggins ML. The thermodynamic properties of liquids included solutions: part 1. Intermolecular energies in mono atomic liquids and their mixtures. J Phys Chem. 1970;74:371–80.
Yadav JS, Singh KC, Sharma VK. Molar excess volumes and excess isentropic compressibilities of 2-methylaniline (i) + benzene (j) + methylbenzene}, {2-methylaniline (i) + benzene (j) + 1,2-dimethylbenzene (k)}, and {2-methylaniline (i) + benzene (j) + 1,4-dimethylbenzene (k) at T) 308.15 K. J Chem Eng Data. 2009;54:2109–12.
Sharma D, Yadav JS, Singh KC, Sharma VK. Molar excess volumes and excess isentropic compressibilities of ternary mixtures containing o-toluidine. J Sol Chem. 2008;37:1099–112.
Jangra SK, Yadav JS, Sharma VK. Thermodynamic investigations of ternary o-toluidine + tetrahydropyran + N, N-dimethylformamide mixture and its binaries at 298.15, 303.15 and 308.15 K. J Mol Liq. 2011;163:36–45.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Kier LB, Yalkowasky SH, Sinkula AA, Valvani SC. Physicochemical properties of drugs. New York: Mercel Dekker; 1980.
Sharma VK, Kataria J, Sharma D. Excess heat capacities of mixtures containing 1-ethyl-3- methylimidazolium tetrafluoroborate, lactams and cyclic alkanones. J Therm Anal Calorim. 2015;121:777–96.
Sharma VK, Dua R, Dimple, Jangra SK. Heat capacities of binary and ternary mixtures containingo-chlorotoluene, cyclic ether and aromatic hydrocarbons. Fluid Phase Equilib. 2014;378:83–92.
Acknowledgements
The authors are thankful to Mr. K. Chandrasekhar Reddy, SSBN College, Anantapur, for providing Gaussian-09 facility, and C-DAC, Pune, India, for providing the computational work. V.K. Sharma is grateful to University Grant Commission (UGC), New Delhi, for the award of SAP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malik, S., Gupta, H. & Sharma, V.K. Excess heat capacities and excess molar enthalpies of the mixtures containing tetrahydropyran, piperidine and cyclic alkanones. J Therm Anal Calorim 132, 1263–1275 (2018). https://doi.org/10.1007/s10973-018-7032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7032-2