Abstract
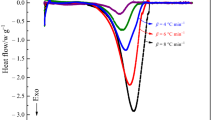
Cosmetic products that contain malic, salicylic, hyaluronic acids and various antioxidants, which are popular worldwide, have made cosmeceuticals a popular cosmetic production sector. Typically, each component of cosmetics, regardless of its activity, may affect the thermal stability of the product. To investigate the thermal stability of regular cosmetics of interest, thermal activity monitor IV was applied to determine the thermokinetic parameters for stability assessment. Arrhenius equations and thermal safety software (for kinetic calculations and numerical simulations) were used. Considering the numerous additives in cosmetic products, individual components of cosmetic material mixed with other components are the primary factors for product deterioration. We examined samples of pure malic acid, pure salicylic acid, and individual acids mixed with copper or iron oxide under isothermal surroundings at 80, 90, 100, 110, and 120 °C. The results of isothermal tests were compared with nonisothermal tests using Arrhenius equations, ASTM E698 method, and Flynn–Wall–Ozawa methods. The value of Ea between malic acid and salicylic acid became lower when mixed with CuO. The findings can be used to identify the optimal parameters for product design and establish a malic and salicylic acid database for developing a proactive loss prevention protocol.


Similar content being viewed by others
References
Iwegbue CM, Emakunu OS, Nwajei GE, Bassey FI, Martincigh BS. Evaluation of human exposure to metals from some commonly used bathing soaps and shower gels in Nigeria. Regul Toxicol Pharmacol. 2017;83:38–45.
Kaličanin B, Velimirović D. A study of the possible harmful effects of cosmetic beauty products on human health. Biol Trace Elem Res. 2016;170(2):476–84.
Shimpi SS. Structural equation modeling for men’s cosmetics behavior research. Indian J Market. 2016;46(7):36–54.
Ingram AL, Nickels TM, Maraoulaite DK, White RL. Thermogravimetry–mass spectrometry investigations of montmorillonite interlayer water perturbations caused by aromatic acid adsorbates. J Therm Anal Calorim. 2016;126(3):1157–66.
Gálico DA, Nova CV, Bannach G. Polymorphism in propyl gallate recrystallized with acetone. J Therm Anal Calorim. 2016;128(1):611–4.
Bezerra GSN, Pereira MAV, Ostrosky EA, Barbosa EG, de Moura MdFV, Ferrari M, Aragão CFS, Gomes APB. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J Therm Anal Calorim. 2016;127(2):1683–91.
Iwegbue CMA. Safety evaluation of the metals in some brands of nail polish in Nigeria. J Verbrauch Lebensm. 2016;11(3):271–8.
Telegdi J, Trif L, Nagy E, Mihály J, Molnár N. New comonomers in malic acid polyesters. J Therm Anal Calorim. 2017;129(2):991–1000.
Van Staden J, Volschenk H, Van Vuuren H, Viljoen-Bloom M. Malic acid distribution and degradation in grape must during skin contact: the influence of recombinant malo-ethanolic wine yeast strains. S Afr J Enol Vitic. 2017;26(1):16–20.
Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25(1):18–22.
Huang AC, Chen WC, Huang CF, Zhao JY, Deng J, Shu CM. Thermal stability simulations of 1,1-bis(tert-butylperoxy)-3,3,5 trimethylcyclohexane mixed with metal ions. J Therm Anal Calorim. 2017;130(2):949–57.
Chen WC, Lin JR, Liao MS, Wang YW, Shu CM. Green approach to evaluating the thermal hazard reaction of peracetic acid through various kinetic methods. J Therm Anal Calorim. 2016;127(1):1019–26.
Willms T, Kryk H, Oertel J, Lu X, Hampel U. Reactivity of t-butyl hydroperoxide and t-butyl peroxide toward reactor materials measured by a microcalorimetric method at 30° C. J Therm Anal Calorim. 2016;128(1):319–33.
Wang Y, Xia X, Zhu J, Li Y, Wang X, Hu X. Catalytic activity of nanometer-sized CuO/Fe2O3 on thermal decompositon of AP and combustion of AP-based propellant. Combust Sci Technol. 2010;183(2):154–62.
Tsai LC, Tsai YT, Lin CP, Liu SH, Wu TC, Shu CM. Isothermal versus non-isothermal calorimetric technique to evaluate thermokinetic parameters and thermal hazard of tert-butyl peroxy-2-ethyl hexanoate. J Therm Anal Calorim. 2012;109(3):1291–6.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52(24):8206–15.
Wang CP, Yang Y, Tsai YT, Deng J, Shu CM. Spontaneous combustion in six types of coal by using the simultaneous thermal analysis-Fourier transform infrared spectroscopy technique. J Therm Anal Calorim. 2016;126(3):1591–602.
Deng J, Zhao JY, Xiao Y, Zhang YN, Huang AC, Shu CM. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J Therm Anal Calorim. 2017;129(3):1779–86.
Whitmore MW, Wilberforce JK. Use of the accelerating rate calorimeter and the thermal activity monitor to estimate stability temperatures. J Loss Prev Process Ind. 1993;6(2):95–101.
Nafisi S, Saboury AA, Keramat N, Neault JF, Tajmir-Riahi HA. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J Mol Struct. 2007;827(1):35–43.
Hou HY, Liao TS, Duh YS, Shu CM. Thermal hazard studies for dicumyl peroxide by DSC and TAM. J Therm Anal Calorim. 2006;83(1):167–71.
Yao J, Tian L, Wang Y, Djah A, Wang F, Chen H, Su C, Zhuang R, Zhou Y, et al. Microcalorimetric study the toxic effect of hexavalent chromium on microbial activity of Wuhan brown sandy soil: an in vitro approach. Ecotoxicol Environ Saf. 2008;69(2):289–95.
Hou HY, Shu CM, Duh YS. Exothermic decomposition of cumene hydroperoxide at low temperature conditions. AIChE J. 2001;47(8):1893–6.
Chiang CL, Liu SH, Lin YC, Shu CM. Thermal release hazard for the decomposition of cumene hydroperoxide in the presence of incompatibles using differential scanning calorimetry, thermal activity monitor III, and thermal imaging camera. J Therm Anal Calorim. 2016;127(1):1061–9.
Hao YH, Huang Z, Ye QQ, Wang JW, Yang XY, Fan XY, Li YL, Peng YW. A comparison study on non-isothermal decomposition kinetics of chitosan with different analysis methods. J Therm Anal Calorim. 2016;128(2):1077–91.
Saha B, Ghoshal A. Thermal degradation kinetics of poly (ethylene terephthalate) from waste soft drinks bottles. Chem Eng J. 2005;111(1):39–43.
Wang J, Jia H, Tang Y, Xiong X, Ding L. Thermal stability and non-isothermal crystallization kinetics of metallocene poly (ethylene–butene–hexene)/high fluid polypropylene copolymer blends. Thermochim Acta. 2017;647:55–61.
Bisinella RZ, Ribeiro JC, de Oliveira CS, Colman TA, Schnitzler E, Masson ML. Some instrumental methods applied in food chemistry to characterise lactulose and lactobionic acid. Food Chem. 2017;220:295–8.
Braud C, Bunel C, Vert M. Poly (β-malic acid): a new polymeric drug-carrier. Polym Bull. 1985;13(4):293–9.
Matyasovszky N, Tian M, Chen A. Kinetic study of the electrochemical oxidation of salicylic acid and salicylaldehyde using UV/vis spectroscopy and multivariate calibration. J Phys Chem A. 2009;113(33):9348–53.
Acknowledgements
This study was funded by the Anhui Province Education Department Natural Sciences Key Fund, China (Grant No. KJ2017A078). The assistance of Prof. Olive J. Hao, YunTech, Feng Tay Chair Professor is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Liu, SH., Huang, AC. et al. Effects of mixing malic acid and salicylic acid with metal oxides in medium- to low-temperature isothermal conditions, as determined using the thermal activity monitor IV. J Therm Anal Calorim 133, 779–784 (2018). https://doi.org/10.1007/s10973-018-7003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7003-7