Abstract
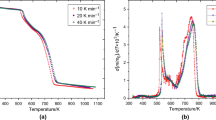
The increasing numbers of used tyres constitute a serious threat to the natural environment. The progress made in recent years in the management of polymer wastes has meant that used tyres are starting to be perceived as a potential source of valuable raw materials. The objective of this research was to study the burning characteristics of various used tyres. Waste tyres of seven producers have been tested. In order to understand thermal properties, three different assessment methods were used to study the behaviour of the material: (1) determination of ash content, (2) flame propagation test and (3) thermal analysis. The ash content test can be used to analyse how many percentage of the tyre leaves in the form of smoke or gas. The flame propagation test gives information on the duration of the combustion and the degree of smoke generation. Results of thermal analysis (TG/DTG/DTA) show the degree and speed of the mass changes of the different tyre types, the enthalpy change and the temperature of the reactions during heating. By combustion tests, it was modelled how the tyres behave when they are burning in incineration plant. In terms of recycling, those tyres are better, which have low decomposition temperature and smaller residual mass. This also means that maximum combustion heat can be recovered. After burning, the samples showed the greatest difference in loss of mass; however, all are different in flammability, afterglow time and their thermal stability.




Similar content being viewed by others
References
Tenk A. Természeti erőforrás és környezetgazdálkodás 5. (in English: Natural Resources Management and Environmental Management 5), Környezetgazdálkodás Alapjai (in English: The Basics of Environmental Management), Chapter 5.6, University of Western Hungary. 2010. http://www.tankonyvtar.hu/hu/tartalom/tamop425/0027_TEK5/ch01s06.html. Downloading date: 09/03/2016, search engine: tankonyvtar.hu, search terms: környezetszennyezés (in English: environmental pollution).
Restás Á. Drone applications for supporting disaster management. World J Eng Technol. 2015;3(3B):316–21.
Janowska G, Kucharska-Jastrząbek A, Rybiński P, Wesołek D, Wójcik I. Flammability of diene rubbers. J Therm Anal Calorim. 2010;102:1043–9.
Rybiński P, Janowska G, Kucharska-Jastrząbek A, Pająk A, Wójcik I, Wesołek D, Bujnowicz K. Flammability of vulcanizates of diene rubbers. J Therm Anal Calorim. 2012;107:1219. https://doi.org/10.1007/s10973-011-1728-x.
Danon B, Görgens J. Determining rubber composition of waste tyres using devolatilisation kinetics. Thermochim Acta. 2015;621:56.
Restás Á. Decision making method in emergency. Pro Publico Bono: Magyar Közigazgatás (in English: Pro Publico Bono: Hungarian Public Service; A Nemzeti Közszolgálati Egyetem Közigazgatás-Tudományi Szakmai Folyóirata). 2014;3:126–136
Ritter SK. Tyre inferno, C&EN Magazine, internet-based article. 2013. http://cen.acs.org/articles/91/i43/Tyre-Inferno.html. Downloading date: 05/04/2016, search engine: google.hu, search terms: 1983 tyre fire Virginia.
Steve. Burn rubber: the world’s 9 worst tyre fires, internet-based article. 2013. http://webecoist.momtastic.com/2013/05/28/burn-rubber-the-worlds-9-worst-tyre-fires/3/. Downloading date: 23/03/2016, search engine: google.hu, search terms: burn rubber.
Smith D. The great Everett tyre fire, 25 years later, internet-based article. 2010. http://www.heraldnet.com/article/20090924/NEWS01/709249870. Downloading date: 05/04/2016, search engine: google.hu, search terms: 1984 Washington tyre fire.
Rowe M. Dumped on, the guardian, internet-based article. 2002. http://www.theguardian.com/society/2002/may/15/environment.waste. Downloading date: 17/04/2016, search engine: google.hu, search terms: 1989 Wales tyre fire.
Warren J. California and the west: tyre fire spews hazardous smoke: pollution: mammoth dump catches fire in northern San Joaquin Valley. Residents are warned to stay indoors, Los Angeles Times. 1999. http://articles.latimes.com/1999/sep/23/news/mn-13314. Downloading date: 17/04/2016, search engine: google.hu, search terms: 1999 California tyre fire.
Miller MA, Large R. Kirby tyre recycling, Inc., Wyandot Country, Ohio EPA. 2006. http://www.epa.state.oh.us/Portals/47/media/Kirby_Factsheet.pdf. Downloading date: 10/04/2016, search engine: google.hu, search terms: 1999 Ohio tyre fire.
Aburawa A. Tyre fire in Kuwait seen from Space, internet-based article. 2012. http://www.greenprophet.com/2012/04/tyre-fire-in-kuwait-seen-from-space/. Downloading date: 14/04/2016, search engine: google.hu, search terms: Kuwait tyre fire.
MTI Archives. 2013. http://archiv1988tol.mti.hu/Pages/HirSearch.aspx?Pmd=1. Downloading date: 05/04/2016, search engine: mti.hu, search terms: Boba fire.
Melbourne Industrial Fire. 2016. http://www.abc.net.au/news/2016-01-11/industrial-fire-sends-thick-smoke-across-melbourne/7080002. Downloading date: 05/04/2016, search engine: google.hu, search terms: tyre fire, Melbourne.
Ginic-Markovic M, Choudhury NR, Dimopoulos M, Williams DR, Matisons J. Characterization of elastomer compounds by thermal analysis. Thermochim Acta. 1998;316:87.
Scuracchio CH, Waki DA, da Silva MLCP. Thermal analysis of ground tire rubber devulcanized by microwaves. J Therm Anal Calorim. 2007;87:893. https://doi.org/10.1007/s10973-005-7419-8.
ISO 3795-1989 Road vehicles, and tractors machinery for agriculture and forestry—determination of burning behaviour of interior materials.
Hansen PA. Fire in tyres. Heat release rate and response of vehicles. SYNTEF Report STF25 A95039. 1995. http://www.risefr.no/media/publikasjoner/upload/stf25-a95039.pdf. Downloading date: 05/04/2016, search engine: google.hu, search terms: tyre fire.
Juma N, Korenova Z, Markos J, Jelemensky L, Bafrnec M. Experimental study of pyrolysis and combustion of scrap tyre. Polym Adv Technol 2007;18(2):144–148. Published online 21 November 2006 in Wiley InterScience (www.interscience.wiley.com). https://doi.org/10.1002/pat.811.
Acknowledgements
Second author acknowledges the support by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerekes, Z., Lublóy, É. & Kopecskó, K. Behaviour of tyres in fire. J Therm Anal Calorim 133, 279–287 (2018). https://doi.org/10.1007/s10973-018-7001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7001-9