Abstract
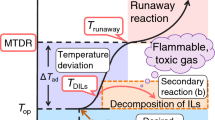
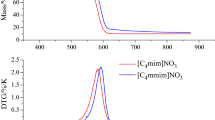
Ionic liquids (ILs) are a relatively new class of environmentally benign and comparatively safe solvents and are expected to have numerous applications in chemical processes. Although pure ILs are thermally stable, the presence of impurities can affect their thermal stability and decomposition behavior. In addition, ILs decomposition products include flammable gases that may present a fire hazard. When designing safer processes and operating conditions, it is therefore important to investigate IL thermal properties and decomposition products in combination with additives. The present work focused on cellulose dissolution which is promising application of ILs to obtain better understanding of thermal hazards. Mixtures of cellulose, iron (III) oxide (Fe2O3), copper(II) oxide (CuO), chromium, and nickel with 1-butyl-3-methylimidazolium chloride (BmimCl) were examined, using differential scanning calorimetry, high-sensitivity calorimetry, and thermogravimetry–differential thermal analysis–mass spectrometry. The addition of CuO was found to generate an exothermic reaction below the decomposition temperature of BmimCl and also to lower the decomposition temperature. BmimCl/CuO mixtures also produced extremely flammable gases below the decomposition temperature of pure BmimCl.










Similar content being viewed by others
References
Zhimin X, Yuwei Z, Xiao-qin Z, Yuanyuan C, Tiancheng M. Thermal stabilities and decomposition mechanism of amino- and hydroxyl-functionalized ionic liquids. Thermochim Acta. 2014;578:59–67.
Martyn JE, Kenneth RS. Ionic liquids. Green solvents for the future. Pure Appl Chem. 2000;72:1391–8.
Ngoc LM, Kihun A, Yoon-Mo K. Methods for recovery of ionic liquids—a review. Process Biochem. 2014;49:872–81.
Pengfei Z, Yutong G, Yiqi L, Yan G, Yong W, Cong W. Ionic liquids with metal chelate anions. Chem Commun. 2012;48:2334–6.
John SW. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002;4:73–80.
Fabio B, Cinzia C. The heck reaction in ionic liquids: progress and challenges. Molecules. 2010;15:2211–45.
Christian PM. Supported ionic liquid catalysis. Chem Eur. 2005;11:50–6.
Roberto IC, Joan FB. Comparison of Ionic liquids to conventional organic solvents for extraction of aromatics from aliphatics. J Chem Eng Data. 2016;61:1685–99.
Samir IAE. Ionic liquids recycling and reuse. In: Scott TH, editor. Ionic liquids—classes and properties. London: Intech; 2011. p. 239–72.
Barbara R, Martin S, Andrea B, Martin W, Wolfgang K. Polymer electrolyte for lithium batteries based on photochemically crosslinked poly(ethylene oxide) and ionic liquid. Eur Polym J. 2008;44:2986–90.
Zhe W, Steen MR, Bradley DG, Timothy AG. Safety concerns in a pharmaceutical manufacturing process using dimethyl sulfoxide (DMSO) as a solvent. Org Process Res Dev. 2012;16:1994–2000.
Francis S. Atsumi Miyake translator. Thermal safety of chemical process: risk assessment and process design. Tokyo: Maruzen; 2011.
Horng-Jang L, Shih-Kai H, Hao-Ying C, Sheng-Nan L. 2012 International symposium on safety science and technology reason for ionic liquids to be combustible. Procedia Engineering, vol. 45; 2012. p. 502–6.
Marek K, Jan G, Jarl BR. Thermal stability of low temperature ionic liquids revisited. Thermochim Acta. 2004;412:47–53.
Helen LN, Karen L, Liesl H, Alan BM. Thermal properties of imidazolium ionic liquids. Thermochim Acta. 2000;357–8:97–102.
Douglas MF, Jeffrey WG, Hugh CDL, Paul CT. TGA decomposition kinetics of 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and the thermal effects of contaminants. J Chem Thermodyn. 2005;37:900–5.
Walid HA, Jeffrey W, Marc N, Richard HH, Thomas E, John C, Paul CT, Hugh CD, Douglas MF. Thermal degradation studies of alkyl-imidazolium salts and their application in nanocomposites. Thermochim Acta. 2004;409:3–11.
Douglas MF, Walid HA, Jeffrey WG, Paul HM, Hugh CDL, Paul CT. Flammability, thermal stability, and phase change characteristics of several trialkylimidazolium salts. Green Chem. 2003;5:724–7.
Qiwei Y, Kun Y, Huabin X, Baogen S, Zongbi B, Yiwen Y, Qilong R. The effect of molecular solvents on the viscosity, conductivity and ionicity of mixtures containing chloride anion-based ionic liquid. J Ind Eng Chem. 2013;19:1708–14.
Frank W, Loredana NT, Frank M. Thermostability of imidazolium ionic liquids as direct solvents for cellulose. Thermochim Acta. 2012;528:76–84.
Yujin C, Rubing Z, Tao C, Jing G, Mo X, Huizhou L. Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives. Appl Microbiol Biotechnol. 2017;101:521–32.
Martin G, Pedro F, Thomas H. Ionic liquids—promising but challenging solvents for homogeneous derivatization of cellulose. Molecular. 2012;17:7458–502.
Richard PS, Scott KS, John DH, Robin DR. Dissolution of cellulose with ionic liquids. J Am Chem Soc. 2002;124:4974–5.
Hyungsup K, Yongjun A, Seung Y. Comparing the influence of acetate and chloride anions on the structure of ionic liquid pretreated lignocellulosic biomass. Biomass Bioenergy. 2016;93:234–53.
Yan C, Jin W, Jun Z, Huiquan L, Yi Z, Jiasong H. Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem Eng J. 2009;147:13–21.
Samira VF, Yong-Wah K, Constance AS. A coupled low temperature oxidative and ionic liquid pretreatment of lignocellulosic biomass. Catal Today. 2016;269:2–8.
Yamamoto Y, Miyake A. Influence of a mixed solvent containing ionic liquids on the thermal hazard of the cellulose dissolution process. J Therm Anal Calorim. 2017;127:743–8.
Chieu DT, Silvia L, Daniel O. Absorption of water by room-temperature ionic liquids: effect of anions on concentration and state of water. Appl Spectrosc. 2003;57:152–7.
Anastasia E, Grit H, Peer S. Thermal stability and crystallization behavior of imidazolium halide ionic liquids. Thermochim Acta. 2013;573:162–9.
United Nations. Globally harmonized system of classification and labeling of chemicals (GHS) fourth revised edition. 2011. https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf. Accessed 25 June 2017.
Maaike CK, Wim B, Cor JP, Geert-Jan W. Quantum chemical aided prediction of the thermal decomposition mechanisms and temperatures of ionic liquids. Thermochim Acta. 2007;465:40–7.
Arindrajit C, Stefan TT. Confined rapid thermolysis/FTIR/ToF studies of imidazolium-based ionic liquids. Thermochim Acta. 2006;443:159–72.
Yan H, Jing P, Shaowen H, Jiuqiang L, Maolin Z. Thermal decomposition of allyl-imidazolium-based ionic liquid studied by TGA–MS analysis and DFT calculations. Thermochim Acta. 2010;501:78–83.
NIST Mass Spec Data Center. Mass Spectra in NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Eds. https://doi.org/10.18434/t4d303. Accessed 21 July 2017.
Siedlecka EM, Czerwicka M, Neumann J, Stepnowski P, Fernández JF, Thöming J. Ionic liquids: methods of degradation and recovery. In: Ionic liquids: theory, properties, new approaches. 2011. http://www.intechopen.com/books/ionic-liquids-theory-properties-new-approaches/ionic-liquids-methods-of-degradation-and-recovery. Accessed 18 July 2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaki, N., Shiota, K., Izato, Yi. et al. Analysis of the thermal hazards of 1-butyl-3-methylimidazolium chloride mixtures with cellulose and various metals. J Therm Anal Calorim 133, 797–803 (2018). https://doi.org/10.1007/s10973-018-6993-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6993-5