Abstract
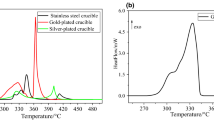
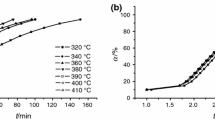
We quantified the thermal stability of 2,2′-azobis(2,4-dimethyl)valeronitrile (ADVN) under isothermal conditions through thermogravimetry (TG), differential scanning calorimetry (DSC), and model-free as well as model-fitting methods. We selected two gaseous atmospheres—N2 and air—to observe the thermal degradation behaviors of ADVN with or without O2 by TG. The results showed that when oxygen was present, the mass loss and thermal degradation rate of ADVN decreased because of azoxy compounds production. In addition, the thermal degradation behaviors of ADVN were noticeable at 30 °C, which is close to room temperature. Therefore, storage and transportation of ADVN should be appropriately controlled under low-temperature conditions. Finally, the kinetic behaviors of ADVN were determined using DSC data, model-free, and model-fitting methods under isothermal conditions.








Similar content being viewed by others
References
Chiang CL, Liu SH, Lin YC, Shu CM. Thermal release hazard for the decomposition of cumene hydroperoxide in the presence of incompatibles using differential scanning calorimetry, thermal activity monitor III, and thermal imaging camera. J Therm Anal Calorim. 2017;127:1061–9.
Yang Y, Tsai YT, Cao CR, Shu CM. Kinetic and thermal safety analysis for tert-butyl peroxy-3,5,5-trimethylhexanoate by advanced calorimetric technology. J Therm Anal Calorim. 2017;127:2253–62.
Porada S, Czerski G, Dziok T, Grzywacz P, Makowska D. Kinetics of steam gasification of bituminous coals in terms of their use for underground coal gasification. Fuel Process Technol. 2015;130:282–91.
Artini C, Nelli L, Pani M, Costa GA, Caratto V, Locardi F. Thermal decomposition of Ce-Sm and Ce-Lu mixed oxalates: influence of the Sm- and Lu-doped ceria structure. Thermochim Acta. 2017;651:100–7.
Chen WC, Lin JR, Liao MS, Wang YW, Shu CM. Green approach to evaluating the thermal hazard reaction of peracetic acid through various kinetic methods. J Therm Anal Calorim. 2017;127:1019–26.
http://news.timedg.com/2012-06/28/10828921.shtml (2011). Accessed 11 July 2017.
Liu SH, Chen YC, Hou HY. Thermal runaway hazard studies for ABVN mixed with acids or alkalines by DSC, TAM III, and VSP2. J Therm Anal Calorim. 2015;122:1107–16.
Zhang T, Xie CX, Jin MP, Sun F, Zhang LL. Study on thermal hazard of ABVN and influence of impurities. Acta Physico Chimica Sinica. 2012;22:122–7.
Wei W (2013) Study on thermal hazards of oil-soluble azo initiators. Nanjing University of Science & Technology, Master Thesis, Nanjing, PR China.
Yang Y, Tsai YT, Zhang Y, Shu CM, Deng J. Inhibition of spontaneous combustion for different metamorphic degrees of coal using Zn/Mg/Al–CO3 layered double hydroxides. Process Saf Environ. 2018;113:401–12.
Li KY, Tsai SY, Lin CP, Tsai YT, Shu CM. Smart technology for evaluating fire Extinguishing effect of tert-butyl hydroperoxide. Ind Eng Chem Res. 2013;52:10969–76.
Tong JW, Chen WC, Tsai YT, Cao Y, Chen JR, Shu CM. Incompatible reaction for (3-4-epoxycyclohexane) methyl-3′-4′-epoxycyclohexyl-carboxylate (EEC) by calorimetric technology and theoretical kinetic model. J Therm Anal Calorim. 2014;116:1445–52.
Wang C, Yang Y, Tsai YT, Deng J, Shu CM. Spontaneous combustion in six types of coal by using the simultaneous thermal analysis-Fourier transform infrared spectroscopy technique. J Therm Anal Calorim. 2016;126:1591–602.
Yang Y, Tsai YT. Evaluation on the photosensitivity of 2,2′-azobis(2,4-dimethyl)valeronitrile with UV. Molecules. 2017;22:2219.
Shu CM, Chang YH, Chiu CW. Evaluation on the thermal stability and hazards behaviors of ADVN using green thermal analysis approach. J Civil Eng. 2016;10:280–90.
Wan W, Chen W, Wei S, Shen Z, Zhang C. Analysis and assessment of the thermal safety of azobisisobutyronitril. China Saf Sci J. 2012;22:131–7.
Lu KM, Lee WJ, Chen WH, Lin TC. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl Energ. 2013;105:57–65.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52:8206–15.
Han Z, Sachdeva S, Papadaki M, Mannan S. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards. Thermochim Acta. 2016;624:69–75.
Šesták J. The quandary aspects of non-isothermal kinetics beyond the ICTAC kinetic committee recommendations. Thermochim Acta. 2015;611:26–35.
Guide Chem, http://www.guidechem.com/reference/dic-12636.html (2015). Accessed 5 May 2017.
Ramírez C, Rico M, Torres A, Barral L, López J, Montero M. Macromolecular nanotechnology-review Epoxy/POSS organic–inorganic hybrids: ATR-FTIR and DSC studies. Eur Polym J. 2008;44:3035–45.
Bacosca L, Hamciuc E, Cristea M, Lisa G, Bruma M. Poly(ether imide)s containing cyano substituents and thin films made from them. Thermochim Acta. 2012;124:1956–66.
Sovizi MR. Thermal behavior of drugs: investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102:285–9.
Alonso MV, Oliet M, Garc´ıa J, Echeverr´ıa J, Oliet M. Gelation and isoconversional kinetic analysis of lignin–phenol–formaldehyde resol resins cure. Chem Eng J. 2006;122:159–66.
Pérez JM, Oliet M, Alonso MV, Rodr´ıguez F. Cure kinetics of lignin–novolac resins studied by isoconversional methods. Thermochim Acta. 2009;487:39–42.
Park SJ, Jin FL. Thermal stabilities and dynamic mechanical properties of sulfone-containing epoxy resin cured with anhydride. Polym Degrad Stabil. 2004;86:515–20.
Li KY, Tsai SY, Lin CP, Tsai YT, Shu CM. Smart technology for evaluating fire extinguishing effect of tert-butyl hydroperoxide. Ind Eng Chem Res. 2013;52:10969–76.
Doyle CD. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal Chem. 1961;22:77–9.
National Institute of Standard and Technology. http://webbook.nist.gov/cgi/cbook.cgi?ID=C78671&Mask=2#ref-4 (2011). Accessed 16 Aug 2017.
Newbold BT. Hydrazo azo, and azoxy groups: part 1. In: Patai S, editor. Oxidation and synthetic uses of hydrazo azo, and azoxy compounds, vol. 1. Chichester: Wiley; 2010. p. 541. ISBN 9780-4716-69265.
Chiu YC, Tsai HC. Thermal and morphology properties of various silica contents in sulfone epoxy nanocomposites. J Appl Polym Sci. 2012;125:E523–31.
Jin FL, Park SJ. Thermal properties of epoxy resin/filler hybrid composites. Polym Degrad Stabil. 2012;97:2148–53.
Liu SH, Kuan CF, Kuan HC, Shen MY, Yang JM, Chiang CL. Preparation and flame retardance of polyurethane composites containing microencapsulated melamine polyphosphate. Polymers. 2017;9:407.
Acknowledgements
Financial support for this work was provided by projects of the China Postdoctoral Science Foundation (No. 2017M610918).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Tsai, YT. Evaluation on thermal stability and kinetics of 2,2′-azobis(2,4-dimethyl)valeronitrile in aerobic and anaerobic conditions under isothermal process. J Therm Anal Calorim 132, 1961–1968 (2018). https://doi.org/10.1007/s10973-018-6980-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6980-x