Abstract
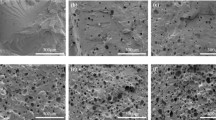
Cross-linked polyurethane (PU) is a promising supporting material for phase change materials (PCMs) because it has excellent physical properties, high impact strength and relatively good chemical resistance. PU prepared from polyethylene glycol can also function as solid–solid PCMs. In this paper, PU/SA composites were synthesized as dual PCMs by entrapping SA into cross-linked PU through in situ polymerization. SA served as a PCM, while cross-linked PU served as both a PCM and supporting material. Fourier transform infrared spectroscopy, differential scanning calorimetry, X-ray diffraction (XRD), polarizing optical microscopy (POM), thermogravimetric analysis (TG) and scanning electron microscopy (SEM) were used to investigate the chemical structure and basic properties of PU/SA composites. The XRD and POM patterns indicate that the synthesized composite has a completely crystalline structure and defective crystallization compared with pristine PEG and SA. The maximum enthalpy of the composite in the heating (cooling) process reaches 167.1 J g−1 (165.4 J g−1), which is 169.35% higher than that of PU. TG results show that the synthesized composites possess good thermal stability. It is clearly that the resulting PU/SA composites are sure to have great potential in thermal energy storage due to their large latent heat, suitable phase change temperature and high thermal stability.










Similar content being viewed by others
References
Pielichowska K, Pielichowski K. Phase change materials for thermal energy storage. Prog Mater Sci. 2014;65:67–123.
Sarier N, Onder E. Organic phase change materials and their textile applications: an overview. Thermochim Acta. 2012;540:7–60.
Chang SJ, Wi S, Jeong S, Kim S. Analysis on phase transition range of the pure and mixed phase change materials (PCM) using a thermostatic chamber test and differentiation. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6603-y.
Li B, Zeng D, Yin X, Chen Q. Theoretical prediction and experimental determination of room-temperature phase change materials using hydrated salts as agents. J Therm Anal Calorim. 2010;100:685–93.
Baetens R, Jelle BP, Gustavsen A. Phase change materials for building applications: a state-of-the-art review. Energ Build. 2010;42:1361–8.
Kim EY, Kim HD. Preparation and properties of microencapsulated octadecane with waterborne polyurethane. J Appl Polym Sci. 2005;96:1596–604.
Tang B, Wang L, Xu Y, Xiu J, Zhang S. Hexadecanol/phase change polyurethane composite as form-stable phase change material for thermal energy storage. Sol Energ Mat Sol C. 2016;144:1–6.
Sarı A, Biçer A, Karaipekli A, Alkan C, Karadag A. Synthesis, thermal energy storage properties and thermal reliability of some fatty acid esters with glycerol as novel solid–liquid phase change materials. Sol Energ Mat Sol C. 2010;94:1711–5.
Alkan C, Canik G, Dünya H, Sarı A. Synthesis and thermal energy storage properties of ethylene dilauroyl, dimyristoyl, and dipalmitoyl amides as novel solid–liquid phase change materials. Sol Energ Mat Sol C. 2011;95:1203–7.
Zhang Y, Zheng X, Wang H, Du Q. Encapsulated phase change materials stabilized by modified graphene oxide. J Mater Chem A. 2014;2:5304–14.
Chen Z, Wang J, Yu F, Zhang Z, Gao X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J Mater Chem A. 2015;3:11624–30.
Karaipekli A, Sarı A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol Energy. 2009;83:323–32.
Wang L, Meng D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl Energ. 2010;87:2660–5.
Fang Y, Yu H, Wan W, Gao X, Zhang Z. Preparation and thermal performance of polystyrene/n-tetradecane composite nanoencapsulated cold energy storage phase change materials. Energ Convers Manag. 2013;76:430–6.
Jiao C, Ji B, Fang D. Preparation and properties of lauric acid–stearic acid/expanded perlite composite as phase change materials for thermal energy storage. Mater Lett. 2012;67:352–4.
Fu X, Kong W, Zhang Y, Jiang L, Wang J, Lei J. Novel solid–solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage. RSC Adv. 2015;5:68881–9.
Liu Z, Fu X, Jiang L, Wu B, Wang J, Lei J. Solvent-free synthesis and properties of novel solid–solid phase change materials with biodegradable castor oil for thermal energy storage. Sol Energ Mat Sol C. 2016;147:177–84.
Karaman S, Karaipekli A, Sarı A, Biçer A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol Energ Mat Sol C. 2011;95:1647–53.
Alkan C, Günther E, Hiebler S, Himpel M. Complexing blends of polyacrylic acid-polyethylene glycol and poly(ethylene-co-acrylic acid)-polyethylene glycol as shape stabilized phase change materials. Energ Convers Manag. 2012;64:364–70.
Yuan Y, Zhang N, Tao W, Cao X, He Y. Fatty acids as phase change materials: a review. Renew Sustain Energy Rev. 2014;29:482–98.
Cao L, Tang Y, Fang G. Preparation and properties of shape-stabilized phase change materials based on fatty acid eutectics and cellulose composites for thermal energy storage. Energy. 2015;80:98–103.
Şentürk SB, Kahraman D, Alkan C, Gökçe I. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohyd Polym. 2011;84:141–4.
Alkan C, Sari A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol Energy. 2008;82:118–24.
Sarı A, Alkan C, Biçer A, Karaipekli A. Synthesis and thermal energy storage characteristics of polystyrene-graft-palmitic acid copolymers as solid–solid phase change materials. Sol Energ Mat Sol C. 2011;95:3195–201.
Yuan Y, Li T, Zhang N, Cao X, Yang X. Investigation on thermal properties of capric–palmitic–stearic acid/activated carbon composite phase change materials for high-temperature cooling application. J Therm Anal Calorim. 2016;124:881–8.
Cao Q, Liu P. Hyperbranched polyurethane as novel solid–solid phase change material for thermal energy storage. Eur Polym J. 2006;42:2931–9.
Su J, Liu P. A novel solid–solid phase change heat storage material with polyurethane block copolymer structure. Energ Convers Manage. 2006;47:3185–91.
Ke H. Morphology and thermal performance of quaternary fatty acid eutectics/polyurethane/Ag form-stable phase change composite fibrous membranes. J Therm Anal Calorim. 2017;129:1533–45.
Chen C, Liu W, Wang H, Peng K. Synthesis and performances of novel solid–solid phase change materials with hexahydroxy compounds for thermal energy storage. Appl Energ. 2015;152:198–206.
Kong Weibo, Xiaowei Fu, Yuan Ye, Liu Zhimeng, Lei Jingxin. Preparation and thermal properties of crosslinked polyurethane/lauric acid composites as novel form stable phase change materials with a low degree of supercooling. RSC Adv. 2017;7:29554–62.
Kong W, Lei Y, Jiang Y, Lei J. Preparation and thermal performance of polyurethane/PEG as novel form-stable phase change materials for thermal energy storage. J Therm Anal Calorim. 2017;130:1011–9.
Liu Z, Wu B, Fu X, Yan P, Yuan Y, Zhou C, Lei J. Two components based polyethylene glycol/thermosetting solid-solid phase change material composites as novel form stable phase change materials for flexible thermal energy storage application. Sol Energ Mat Sol C. 2017;170:197–204.
Sarier N, Onder E. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim Acta. 2007;454:90–8.
Song S, Dong L, Zhang Y, Chen S, Li Q, Guo Y, Deng S, Si S, Xiong C. Lauric acid/intercalated kaolinite as form-stable phase change material for thermal energy storage. Energy. 2014;76:385–9.
He Y, Zhang X, Zhang Y, Song Q, Liao X. Utilization of lauric acid-myristic acid/expanded graphite phase change materials to improve thermal properties of cement mortar. Energ Build. 2016;133:547–58.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, B., Fu, W., Kong, B. et al. Preparation and characterization of stearic acid/polyurethane composites as dual phase change material for thermal energy storage. J Therm Anal Calorim 132, 907–917 (2018). https://doi.org/10.1007/s10973-018-6977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6977-5