Abstract
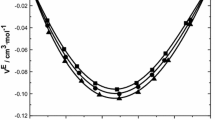
Densities (ρ), speeds of sound (u) and viscosities (η) are reported for binary mixtures of 2-methoxyaniline with carboxylic acids (ethanoic acid, propanoic acid and butanoic acid) over the entire composition range of mole fraction at T = (303.15–318.15) K and at atmospheric pressure 0.1 MPa. The excess properties such as excess molar volume, excess isentropic compressibility and deviation in viscosity are calculated from the experimental density, speed of sound and viscosity. Excess properties are correlated using the Redlich–Kister polynomial equation. The excess partial molar volumes and excess partial molar isentropic compressibilities are calculated for all the binary systems throughout the composition range and at infinite dilutions. The results are analyzed in terms of dipole–dipole interactions and hydrogen bonding between 2-methoxyaniline and carboxylic acid molecules. The VE results are analyzed in the light of Prigogine–Flory–Patterson theory. Analysis of each of the three contributions, viz interactional, free volume and P* to VE, has shown that interactional contribution is positive for all systems, and the free volume and P* contributions are negative for all the binary mixtures. A good agreement is obtained between excess quantities and spectroscopic data.








Similar content being viewed by others
References
Oswal SL, Desai HS. Studies of viscosity and excess molar volume of binary mixtures. Propylamine + 1-alkanol mixtures at 303.15 and 313.15 K. Fluid Phase Equilib. 1998;149:359–76.
Garcia B, Alcalde B, Leal JM, Matos JS. Formamide + (C1–C5) alkan-1-ols solvent systems. J Chem Soc Faraday Trans. 1996;92:3347–52.
Das M, Roy MN. Studies on thermodynamic and transport properties of binary mixtures of acetonitrile with some cyclic ethers at different temperatures by volumetric, viscometric, and interferometric techniques. J Chem Eng Data. 2006;51:2225–32.
Sewnarain R, Raal JD, Ramjugernath D. Isobaric vapor−liquid equilibria for the systems propionic acid + butyric acid, isobutyric acid + butyric acid, butyric acid + isovaleric acid, and butyric acid + hexanoic acid at 14 kPa. J Chem Eng Data. 2002;47:603–7.
Pereiro AB, Rodriguez A. Measurement and correlation of (liquid + liquid) equilibrium of the azeotrope (cyclohexane + 2-butanone) with different ionic liquids at T = 298.15 K. J Chem Thermodynamics. 2008;40:1282–9.
Toumi A, Bouanz M. Volumetric and refractive index properties of isobutyric acid- water binary mixtures at temperatures ranging from 300.15 to 313.15 K. J Mol Liq. 2008;139:55–60.
Abdulagatov IM, Tekin A, Safarov J, Shahverdiyev A, Hassel A. Densities and excess, apparent, and partial molar volumes of binary mixtures of BMIMBF4 + ethanol as a function of temperature, pressure, and concentration. Int J Thermophys. 2008;29:505–33.
Mukesh B, Gowri Sankar M, Chandra Shekar M, Srikanth T. Effect of placement of hydroxyl groups in isomeric butanols on the behavior of thermophysical and spectroscopic properties of 2-methoxyaniline. J Soln Chem. 2015;44:2267–96.
Letcher TM. Excess enthalpies and volumes for mixtures of (Acetonitrile + a carboxylic acid) at 298.15 K. J Chem Eng Data. 2000;45:57–60.
Apelblat A, Manzurola E. Excess molar volumes of carboxylic acid mixtures: binary mixtures of formic, acetic, propionic, n-pentanoic and iso-pentanoic acids at 298.15 K. Fluid Phase Equilib. 1987;38:274–5.
Gupta M, Vibhu I, Shukla JP. Refractive index, molar refraction deviation and excess molar volume of binary mixtures of 1,4-dioxane with carboxylic acids. Phys Chem Liq. 2010;48:415–27.
Cases AM, Marigliano ACG, Solemo HN. Excess molar volume, viscosity and refractive index deviations for mixtures of formamide þ some carboxylic acids at several temperatures. Phys Chem Liq. 2003;41:503–8.
Vogel AL. Text book of practical organic chemistry. London: Longman Green; 1989.
Riddick JA, Bunger W. Organic solvents-physical properties and method of purifications, vol. 2. New York: Wiley Interscience; 1986.
Kumar S, Jeevanandham P. Densities, viscosities, refractive indices and excess properties of aniline and o-anisidine with 2-alkoxyethanols at 303.15 K. J Mol Liq. 2012;174:34–41.
Singh S, Bahadur I, Redhi GG, Ebenso EE, Ramjugernath D. Density and speed of sound of 1-ethyl-3-methylimidazolium ethyl sulphate with acetic or propionic acid at different temperatures. J Mol Liq. 2014;199:518–23.
Vong WT, Tsai FN. Densities, molar volumes, thermal expansion coefficients, and isothermal compressibilities of organic acids from 293.15 K to 323.15 K and at pressures up to 25 MPa. J Chem Eng Data. 1997;42:1116–20.
Gonza´lez B, Dominguez A, Tojo J. Dynamic viscosities, densities, and speed of sound and derived properties of the binary systems acetic acid with water, methanol, ethanol, ethyl acetate and methyl acetate at T = (293.15 298.15 and 303.15) K at atmospheric pressure. J Chem Eng Data. 2004;49:1590–6.
Antina EV, Vyugin AI, Krestov GA. Volume and viscosity characteristics of tetraphenylporfirine solutions at different temperatures. Viniti 1986;1–17.
Antina EV, Vyugin AI, Krestov GA. Polythermic study of density and viscosity of tetra (tert-butyl) tetraphenylporforin. Viniti 1988;1–9.
Bahadur I, Deenadayalu N, Naidoo P, Ramjugernath D. Density, speed of sound, and refractive index measurements for the binary systems (butanoic acid + propanoic acid, or 2-methyl-propanoic acid) at T = (293.15to313.15) K. J Chem Thermodyn. 2013;57:203–11.
Bahadur I, Naidoo P, Singh S, Ramjugernath D, Deenadayalu N. Effect of temperature on density, sound velocity, refractive index and their derived properties for the binary systems (heptanoic acid + propanoic or butanoic acids). J Chem Thermodyn. 2014;78:7–15.
Chandra Sekhar M, Venkatesulu A, Gowrisankar M, Srinivasa Krishna T. Thermodynamic study of interactions in binary liquid mixtures of 2-Chloroaniline with some carboxylic acids. Phys Chem Liq. 2017;55:196–217.
Bhanuprakesh P, Narasimha Rao C, Sivakumar K. Evaluation of molecular interactions by volumetric and acoustic studies in binary mixtures of the ionic liquid [EMIM][MeSO4] with ethanoic and propanoic acid at different temperatures. J Mol Liq. 2016;219:79–87.
Yang CS, Ma PS, Tang DQ, Yin QX, Zhao CW. Excess molar volume, viscosity and heat capacity for the binary mixture of p-xylene and acetic acid at different temperatures. J Chem Eng. 2002;10:604–9.
Zábranský M, Vlastimil Růžička JR. Estimation of the heat capacities of organic liquids as a function of temperature using group additivity. An amendment. J Phys Chem Ref Data. 2004;33:1071–81.
Parveen S, Singh S, Shukla D, Yasmin M, Gupta M, Shukla JP. Study of molecular interactions in binary mixtures of aniline with carboxylic acids at 293.15, 303.15 and 313.15 K. J Soln Chem. 2012;41:156–72.
Cases AM, Gomez Marigliano AC, Bonatti CM, Solimo HN. Density, viscosity, and refractive index of formamide, three carboxylic acids, and formamide + carboxylic acid binary mixtures. J Chem Eng Data. 2001;46:712–5.
Ahluwalia R, Ritu Gupta JL, Vashisht K. Wanchoo, Thermophysical properties of binary liquid systems of ethanoic acid, propanoic acid, and butanoic acid with benzene or acetophenone, ISRN Phys Chem ArticleID 612837, 2013; 2013: 1–13.
Benson GC, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem Thermodyn. 1979;11:1061–7.
Redlich O, Kister AT. Thermodynamics of non electrolytic solutions. Algebraic representation of thermodynamic properties and the classification of solutions. J Ind Eng Chem. 1948;40:345–8.
Canosa J, Rodríguez A, Tojo J. Liquid − liquid equilibrium and physical properties of the ternary mixture (dimethyl carbonate + methanol + cyclohexane) at 298.15 K. J Chem Eng Data. 2001;46:846–50.
Trenjado JL, Matos JS, Alcalde R. Volumetric properties and viscosities of the methyl butanoate + n-heptane + cyclo-octane ternary system at 283.15 and 313.15 K and its binary constituents in the temperature range from 283.15 to 313.15 K. Fluid Phase Equilib. 2002;200:295–315.
Venkateswara Rao P, Gowrisankar M, Venkatramana L, Srinivasa Krishna T, Ravindhranath K. Studies on the importance of nature of substituent on the thermodynamic and transport properties of liquid mixtures at various temperatures. J Chem Thermodyn. 2016;101:92–101.
Wang H, Liu W, Huang J. Densities and volumetric properties of a (xylene + dimethyl sulfoxide) at temperature from (293.15 to 353.15) K. J Chem Thermodyn. 2004;36:743–52.
Hawrylak B, Gracie K, Palepu R. Thermodynamic properties of binary mixtures of butanediols with water. J Soln Chem. 1998;27:17–31.
Patterson D, Delmas G. Corresponding states theories and liquid models. Discuss Faraday Soc. 1970;49:98–105.
Flory PJ. Fifteenth spiers memorial lecture. Thermodynamics of polymer solutions. Discuss Faraday Soc. 1970;49:7–29.
I. Prigogine, R. Defay, in Chemical thermodynamics, 5th ed. London: Longman; 1969. p. 8.
Acknowledgements
The authors are thankful to M/s Anton Paar, Hyderabad, for providing the research facilities and the Managements of Vignan Institute of Technology & Science, Hyderabad, and J.K.C College, Guntur, for their encouragement toward the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukesh, B., Gowrisankar, M., Srinivasa Krishna, T. et al. Studies on the importance of thermodynamic and transport properties of liquid mixtures at various temperatures. J Therm Anal Calorim 132, 1167–1181 (2018). https://doi.org/10.1007/s10973-018-6972-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6972-x