Abstract
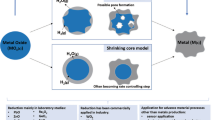
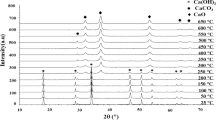
Carbothermic reduction of chromite in the presence of nickel as the alloying element was investigated in a wide temperature range up to 1500 °C using thermogravimetric analysis coupled with continuous off-gas analysis (TG-DSC-MS). Both isothermal and non-isothermal linear heating tests were performed for the kinetic study with the calculation of activation energies. In order to further elucidate the reduction mechanism, the reduced products were characterized by SEM–EDS and XRD. It was concluded that the reduction sequence followed a multi-stage mechanism, reflected partly by the dependency of the activation energy on the extent of reduction. With the progress of reduction, refractory oxide layers gradually formed on/close to the surface of each chromite particle, causing the shift of the rate-limiting factor from chemical control to diffusion control. The promoting effect from the addition of Ni was evident at temperatures higher than 1300 °C due to the formation of alloys of lower melting point.


















Similar content being viewed by others
References
Slatter DD. Technological trends in chromium unit production and supply. INFACON 7; FFF, Trondheim, Norway; 1995. p. 249–62.
Murthy YR, Tripathy SK, Kumar CR. Chrome ore beneficiation challenges & opportunities—a review. Miner Eng. 2011;24:375–80.
Johnson J, Reck BK, Wang T, Graedel TE. The energy benefit of stainless steel recycling. Energy Policy. 2008;36:181–92.
Chakraborty D, Ranganathan S, Sinha SN. Investigations on the carbothermic reduction of chromite ores. Metall Mater Trans B. 2005;36B:437–44.
Niayesh MJ, Dippenaar RJ. The solid state reduction of chromite. In: INFACON 6. Proceedings of the 6th international ferroalloy congress, Cape Town, South Africa; 1992. p. 57–63.
Ding YL, Warner NA. Kinetics and mechanism of reduction of carbon-chromite composite pellets. Ironmak Steelmak. 1997;24(3):224–9.
Perry KPD, Finn CWP, King RP. An ionic diffusion mechanism of chromite reduction. Metall Trans B. 1988;19B:677–84.
Roschin AV, Karnoukhov VN, Roschin VE, Malkov NV. New findings in research of solid phase reactions in chromite ore reduction processes. In: Proceedings: tenth international ferroalloys congress, Cape Town, South Africa; 2004. p. 333–42.
Atasoy A, Sale FR. An investigation on the solid state reduction of chromite concentrate. Solid State Phenom. 2009;147–149:752–7.
Murti NSS, Seshadri V. Kinetics of reduction of synthetic chromite with carbon. Trans ISIJ. 1982;22:925–33.
Soykan O, Eric RH, King RP. The reduction mechanism of a natural chromite at 1416 °C. Metall Trans B. 1991;22B:53–63.
Wang Y, Wang L, Xu J, Chou KC. Kinetics of carbothermic reduction of synthetic chromite. J Min Metall Sect B Metall. 2014;50(1):15–21.
Hu X, Okvist LS, Yang Q, Bjorkman B. Thermogravimetric study on carbothermic reduction of chromite ore under non-isothermal conditions. Ironmak Steelmak. 2015;42(6):409–16.
Nafziger RH, Tress JE, Paige JI. Carbothermic reduction of domestic chromites. Metall Trans B. 1979;10B:5–14.
Kekkonen M, Xiao Y, Holappa L. Kinetic study on solid state reduction of chromite pellets. In: INFACON 7, Trondheim, Norway; 1995. p. 351–60.
Dawson NF. The solid state reduction of chromite (PhD thesis). Durban: University of Natal; 1989.
Dawson NF, Edwards RI. Factors affecting the reduction of chromite. Reio De Janeiro, Barzil: INFACON; 1986. p. 1–11.
Zhao B, Hayes PC. Effects of oxidation on the microstructure and reduction of chromite pellets. In: The twelfth international ferroalloys congress (INFACON XII), Helsinki, Finland; 2010. p. 263–73.
Kleynhans ELJ, Neizel BW, Beukes JP, Zyl PGV. Utilisation of pre-oxidized ore in the pelletised chromite pre-reduction process. Miner Eng. 2016;92:114–24.
Ding YL, Warner NA. Catalytic reduction of carbon-chromite composite pellets by lime. Thermochim Acta. 1997;292:85–94.
Apaydin F, Atasoy A, Yildiz K. Effect of mechanical activation on the carbothermal reduction of chromite with metallurgical coke. Can Metall Q. 2011;50(2):113–8.
Lekatou A, Walker RD. Effect of SiO2 addition on solid state reduction of chromite concentrate. Ironmak Steelmak. 1997;24(2):133–43.
Weber P, Eric RH. Solid-state fluxed reduction of LG-6 chromite from the Bushveld complex. In: INFACON 6. Proceeding of the 6th international ferroalloys congress, Cape Town, South Africa; 1992. p. 71–7.
Weber P, Eric RH. The reduction mechanism of chromite in the presence of a silica flux. Metall Trans B. 1992;24(6):987–95.
Weber P, Eric RH. The reduction of chromite in the presence of silica flux. Miner Eng. 2006;19:318–24.
Duong HV, Johnston RF. Kinetics of solid state silica fluxed reduction of chromite with coal. Ironmak Steelmak. 2000;27(3):202–6.
Deventer JSJV. The effect of additives on the reduction of chromite by graphite: an isothermal kinetic study. Thermochim Acta. 1988;127:25–35.
Hu X, Teng L, Wang H, Okvist LS, Yang Q, Bjorkman B, et al. Carbothermic reduction of synthetic chromite with/without the addition of iron powder. ISIJ Int. 2016;56(12):2147–55.
Hu X, Wang H, Teng L, Seetharaman S. Direct chromium alloying by chromite ore with the presence of metallic iron. J Min Metall Sect B Metall. 2013;49(2):207–15.
Hu X, Yang Q, Okvist LS, Bjorkman B. Thermal analysis study on the carbothermic reduction of chromite ore with the addition of mill scale. Steel Res Int. 2015;86:1–9.
Yape EO. Fe–Ni–Cr crude alloy production from direct smelting of chromite and laterite ores. J Med Bioeng. 2014;3(4):245–50.
Katayama HG, Tokuda M, Ohtani M. Promotion of the carbothermic reduction of chromite ore by the addition of borates. Iron Steel Inst Jpn. 1986;72(10):1513–20.
Bale CW, Belisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, et al. FactSage Thermochemical Software and Database, 2010–2016. Calphad. 2016;54:35–53.
Dresler W, McLean A. The extraction of ferrochromium in the presence of nickel from bird river chromite ores. Can Metall Q. 1992;31(3):181–8.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Phillips WR. A differential thermal study of the chlorites. Mineral Mag. 1963;33(260):404–14.
Acknowledgements
The following contributions are acknowledged: Judith Price for the preparation of polished sections, Derek Smith for XRD analyses, Elizabeth Houghton and Khushmeet Gill for SEM analyses, and KWG Resources Inc. for providing the ore samples. The study was funded by NRCan under the Rare Earth Elements and Chromite R&D Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, D., Paktunc, D. Kinetics and mechanisms of the carbothermic reduction of chromite in the presence of nickel. J Therm Anal Calorim 132, 143–154 (2018). https://doi.org/10.1007/s10973-017-6936-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6936-6