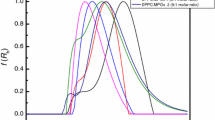
Abstract
Differential scanning calorimetry and synchrotron X-ray diffraction techniques have been employed to investigate the interaction of paeonol with DPPC liposomes and to investigate the location of paeonol molecules in liposomes. The results showed that the location of the paeonol molecules in liposomes is concentration dependent. When the concentration of the paeonol is not more than 5 mol%, both main transition temperature and transition enthalpy of liposomes decrease with increasing the concentration of paeonol, indicating that paeonol molecules incorporate into the hydrophobic region of DPPC molecules. When the concentration of the paeonol is between 5 and 15 mol%, additional paeonol molecules will incorporate into the hydrophilic region of the DPPC molecules and interact with its polar groups, resulting in an increase tendency of the main transition enthalpy. When the concentration of the paeonol is more than 15 mol%, both main transition temperature and transition enthalpy of liposomes decrease with increasing the concentration of paeonol, indicating that the additional paeonol molecules locate at the region of hydrocarbon chains of DPPC again. Calorimetric data show that the main transition temperature of all paeonol/DPPC liposomes is lower than that of pure DPPC liposomes but the enthalpy of liposomes containing more than 10 mol% paeonol is higher than that of pure DPPC liposome, which is related to the nonsynchronous change of the head and tail part of DPPC molecules during the main transition. This study will play an important role in the further investigation of interaction of drugs with biomimetic membranes and in the further study of phase transition mechanisms of DPPC liposome.







Similar content being viewed by others
Abbreviations
- DPPC:
-
Dipalmitoylphosphatidylcholine
- XRD:
-
X-ray diffraction
- DSC:
-
Differential scanning calorimetry
- SAXS:
-
Small angle X-ray scattering
- WAXS:
-
Wide angle X-ray scattering
- L β′ :
-
Lamellar-gel phase
- P β′ :
-
Rippled gel phase
- L α :
-
Liquid–crystal phase
- T p :
-
Temperature of the peak maximum of DSC peak
- ΔT 1/2 :
-
The half-height width of DSC peak
References
Ishiguro K, Ando T, Maeda O, Hasegawa M, Kadomatsu K, Ohmiya N, Niwa Y, Xavier R, Goto H. Paeonol attenuates TNBS-induced colitis by inhibiting NF-κB and STAT1 transactivation. Toxicol Appl Pharm. 2006;217:35–42.
Zong SY, Pu YQ, Xu BL, Zhang T, Wang B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with TNBS-induced ulcerative colitis. Int Immunopharm. 2017;42:32–8.
Li M, Tan SY, Wang XF. Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE2 synthesis and COX-2 expression. Oncol Rep. 2014;32:2845–53.
Fan HY, Qi D, Yu C, Zhao F, Liu T, Zhang ZK, Yang MY, Zhang LM, Chen DQ, Du Y. Paeonol protects endotoxin-induced acute kidney injury: potential mechanism of inhibiting TLR4-NF-κB signal pathway. Oncotarget. 2016;7:39497–510.
Wu RG, Dai JD, Wu FG, Zhang XH, Li WF, Wang YR. Competitive molecular interaction among paeonol-loaded liposomes: differential scanning calorimetry and synchrotron X-ray diffraction studies. Int J Pharm. 2012;438:91–7.
Wu RG, Wang YR, Wu FG, Zhou HW, Zhang XH, Hou JL. A DSC study of paeonol-encapsulated liposomes, comparison of the effect of cholesterol and stigmasterol on the thermotropic phase behavior of liposomes. J Therm Anal Calorim. 2012;109:311–6.
Fielding RM. Liposomal drug delivery: advantages and limitations from a clinical pharmacokinetic and therapeutic perspective. Clin Pharmacokinet. 1991;21:155–64.
Wang ML, Zhao TT, Liu YP, Wang QQ, Xing SS, Li L, Wang LG, Liu LX, Gao DW. Ursolic acid liposomes with chitosan modification: promising antitumor drug delivery and efficacy. Mat Sci Eng C Mater. 2017;71:1231–40.
Ulrich AS. Biophysical aspects of using liposomes as delivery vehicles. Biosci Rep. 2002;22:129–50.
Mohapatra M, Mishra AK. 1-Naphthol as a sensitive fluorescent molecular probe for monitoring the interaction of submicellar concentration of bile salt with a bilayer membrane of DPPC, a lung surfactant. J Phys Chem B. 2010;114:14934–40.
Gmajner D, Ulrih NP. Thermotropic phase behaviour of mixed liposomes of archaeal diether and conventional diester lipids. J Therm Anal Calorim. 2011;106:255–60.
Wu FG, Yang P, Zhang C, Han XF, Song MH, Chen Z. Investigation of drug-model cell membrane interactions using sum frequency generation vibrational spectroscopy: a case study of chlorpromazine. J Phys Chem C. 2014;118:17538–48.
Wu FG, Yang P, Zhang C, Li BL, Han XF, Song MH, Chen Z. Molecular interactions between amantadine and model cell membranes. Langmuir. 2014;30:8491–9.
Li BL, Wang HY, Feng PY, Han XF, Chen Z, Lu XL, Wu FG. Qualitative and quantitative analyses of the molecular-level interaction between memantine and model cell membranes. J Phys Chem C. 2015;119:17074–83.
Jiang YW, Gao G, Chen Z, Wu FG. Fluorescence studies on the interaction between chlorpromazine and model cell membranes. N J Chem. 2017;41:4048–57.
Chiu MH, Prenner EJ. Differential scanning calorimetry: an invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J Pharm Bioallied Sci. 2011;3:39–59.
Dong YD, Boyd BJ. Applications of X-ray scattering in pharmaceutical science. Int J Pharm. 2011;417:101–11.
Bildstein L, Pili B, Marsaud V, Wack S, Meneau F, Lepêtre-Mouelhi S, Desmaële D, Bourgaux C, Couvreur P, Dubernet C. Interaction of an amphiphilic squalenoyl prodrug of gemcitabine with cellular membranes. Eur J Pharm Biopharm. 2011;79:612–20.
Dai WT, Zhang DR, Duan CX, Jia LJ, Wang YC, Feng FF, Zhang Q. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul. 2010;27:234–41.
Holopainen JM, Lemmich J, Richter F, Mouritsen OG, Rapp G, Kinnunen PKJ. Dimyristoylphosphatidylcholine/C16:0-Ceramide binary liposomes studied by differential scanning calorimetry and wide- and small-angle X-ray scattering. Biophys J. 2000;78:2459–69.
Hatta I, Ohta N, Inoue K, Yagi N. Coexistence of two domains in intercellular lipid matrix of stratum corneum. Biochim Biophys Acta. 2006;1758:1830–6.
Pili B, Bourgaux C, Meneau F, Couvreur P, Ollivon M. Interaction of an anticancer drug, gemcitabine, with phospholipid bilayers. J Therm Anal Calorim. 2009;98:19–28.
Yu ZW, Quinn PJ. Phase stability of phosphatidylcholines in dimethylsulfoxide solutions. Biophys J. 1995;69:1456–63.
Wu FG, Jia Q, Wu RG, Yu ZW. Regional cooperativity in the Phase transitions of dipalmitoylphosphatidylcholine bilayers: the lipid tail triggers the isothermal crystallization process. J Phys Chem B. 2011;115:8559–68.
Wu FG, Wang NN, Tao LF, Yu ZW. Acetonitrile induces nonsynchronous interdigitation and dehydration of dipalmitoylphosphatidylcholine bilayers. J Phys Chem B. 2010;114:12685–91.
Longo E, Ciuchi F, Guzzi R, Rizzuti B, Bartucci R. Resveratrol induces chain interdigitation in DPPC cell membrane model systems. Colloids Surf B. 2016;148:615–21.
McMullen TPW, McElhaney RN. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim Biophys Acta. 1995;1234:90–8.
Goodwin GC, Hammond K, Lyle I, Jones MN. Lectin-mediated agglutination of liposomes containing glycophorin. Effects of acyl chain length. Biochim Biophys Acta. 1982;689:80–8.
Wu RG, Chen L, Yu ZW, Quinn PJ. Phase diagram of stigmasterol-dipalmitoylphosphatidylcholine mixtures dispersed in excess water. Biochim Biophys Acta. 2006;1758:764–71.
Arrowsmith M, Hadgraft J, Kellaway IW. The interaction of cortisone esters with liposomes as studied by differential scanning calorimetry. Int J Pharm. 1983;16:305–18.
Quinn PJ. Characterisation of clusters of a-tocopherol in gel and fluid phases of dipalmitoylglycerophosphocholine. Eur J Biochem. 1995;233:916–25.
Wolfe DH, Lis LJ, Kucuk O, Westerman MP, Cunningham BA, Qadri SB, Bras W, Quinn PJ. Phase transitions between ripple structures in hydrated phosphatidylcholine-cholesterol multilamellar assemblies. Phys Rev Lett. 1992;68:1085–8.
Cong WJ, Liu QF, Liang QL, Wang YM, Luo GA. Investigation on the interactions between pirarubicin and phospholipids. Biophys Chem. 2009;143:154–60.
Bakonyi M, Berkó S, Budai-Szűcs M, Kovács A, Csányi E. Differential scanning calorimetry for evaluating the encapsulation efficiency of lidocaine loaded liposomes compared to the ultracentrifugation method. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6394-1.
Matuoka S, Kato S, Hatta I. Temperature change of the ripple structure in fully hydrated dimyristoylphosphatidylcholine/cholesterol multibilayers. Biophys J. 1994;67:728–36.
Halling KK, Slotte JP. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim Biophys Acta. 2004;1664:161–71.
McKersie D, Thompson JE. Influence of plant sterols on the phase properties of phospholipid bilayers. Plant Physiol. 1979;63:802–5.
Pili B, Bourgaux C, Amenitsch H, Keller G, Lepêtre-Mouelhi S, Desmaële D, Couvreur P, Ollivon M. Interaction of a new anticancer prodrug, gemcitabine–squalene, with a model membrane: coupled DSC and XRD study. BBA Biomembr. 2010;1798:1522–32.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 81773916), by the grants of Beijing Natural Science Foundation (Grant No. 7153171), and by the Fundamental Research Funds of Beijing University of Chinese Medicine (2017-JYB-JS-155, 2017-JYB-XS-093). Prof. Dr. Yu ZW (Department of Chemistry, Tsinghua University) is gratefully acknowledged for expert assistance in the DSC and XRD experiments. Assistance of Dr. Li ZH and Dr. Mo G from Beijing Synchrotron Radiation Facility (BSRF) in the synchrotron station facility setup is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, TT., Sun, HY., Deng, G. et al. The interaction of paeonol with DPPC liposomes. J Therm Anal Calorim 132, 685–692 (2018). https://doi.org/10.1007/s10973-017-6894-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6894-z