Abstract
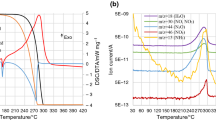
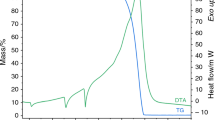
Ammonium nitrate (AN) can decompose and further detonate under some conditions, e.g., in the presence of impurities which act as promoters. Its reactive hazards have been widely studied for several years. However, many large accidents involving AN still happened frequently in the recent years. It is found that, for accidents in storage or transportation, the thermal runaway is a major factor. The main objective of the research is to examine the effect of potassium chloride (KCl) on the thermal stability of AN under acidic conditions. The thermal stability of samples was investigated by differential scanning calorimetry (DSC), accelerating rate calorimetry (ARC), the Cook-off test and the Dewar test. The results indicate that KCl can significantly reduce the thermal stability of AN under acidic conditions. The catalytic effect of KCl on the decomposition of AN is limited without acidic conditions. The scale effect also is important factor to influence the thermal stability of AN.










Similar content being viewed by others
References
Lu CX, Liu ZL, Ni OQ. Theory of industrial explosives. Beijing: Arms Industry Press; 1994.
Wu Q, Tan L, Xu S, Liu D, Min L. Study on thermal decomposition characteristics of ammonium nitrate emulsion explosive in different scales. J Energ Mater. 2017;2:1–9.
Shiota K, Matsunaga H, Miyake A. Effects of amino acids on solid-state phase transition of ammonium nitrate. J Therm Anal Calorim. 2017;127(1):851–6.
Chaturvedi S, Dave PN. Review on thermal decomposition of ammonium nitrate. J Energ Mater. 2013;31(1):1–26.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;67(3):253–81.
Tan L, Xia LH, Wu QJ, Xu S, Liu DB. Detonation characteristics of ammonium nitrate and activated fertilizer mixtures. Combust Explos Shock Waves. 2016;52(3):335–41.
Popławski D, Hoffmann J, Hoffmann K. Effect of carbonate minerals on the thermal stability of fertilisers containing ammonium nitrate. J Therm Anal Calorim. 2016;124(3):1561–74.
Tan L, Xia LH, Wu QJ, Xu S, Liu DB. Effect of urea on detonation characteristics and thermal stability of ammonium nitrate. J Loss Prev Process Ind. 2015;38:169–75.
Vargeese AA, Joshi SS, Krishnamurthy VN. Effect of method of crystallization on the IV–III and IV–II polymorphic transitions of ammonium nitrate. J Hazard Mater. 2009;161(1):373–9.
Wu HB, Chan CK. Effects of potassium nitrate on the solid phase transitions of ammonium nitrate particles. Atmos Environ. 2008;42(2):313–22.
Mousaviazar A, Keshavarz MH, Hayaty M. The effect of cellulose derivatives on the phase transition and thermal behavior of ammonium nitrate. J Therm Anal Calorim. 2017;128(2):1049–56.
Han Z, Sachdeva S, Papadaki MI, Mannan MS. Ammonium nitrate thermal decomposition with additives. J Loss Prev Process Ind. 2015;35:307–15.
Klimova I, Kaljuvee T, Türn L, Bender V, Trikkel A, Kuusik R. Interactions of ammonium nitrate with different additives. J Therm Anal Calorim. 2011;105(1):13.
Laboureur DM, Han Z, Harding BZ, Pineda A, Pittman WC, Rosas C, Jiang J, Mannan MS. Case study and lessons learned from the ammonium nitrate explosion at the West Fertilizer facility. J Hazard Mater. 2016;308:164–72.
Pittman W, Han Z, Harding B, Rosas C, Jiang J, Pineda A, Mannan MS. Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years. J Hazard Mater. 2014;280:472–7.
Kletz TA. Accident reports may not tell us everything we need to know. J Loss Prev Process Ind. 2009;22(6):753–6.
Dechy N, Bourdeaux T, Ayrault N, Kordek MA, Le Coze JC. First lessons of the Toulouse ammonium nitrate disaster, 21st September 2001, AZF plant, France. J Hazard Mater. 2004;111(1):131–8.
Kajiyama K, Izato YI, Miyake A. Thermal characteristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;113(3):1475–80.
Tan L, Wu Q, Chen X, Jiang W, Xu S, Liu D. The effects of sodium chloride on the explosive performance of ammonium nitrate. J Therm Anal Calorim. 2014;115(2):1759–66.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;127(1):204–10.
Wood BJ, Wise H. Acid catalysis in the thermal decomposition of ammonium nitrate. J Chem Phys. 1955;23(4):693–6.
Oxley JC, Smith JL, Rogers E, Yu M. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384(1):23–45.
Keenan AG, Dimitriades B. Mechanism for the chloride-catalyzed thermal decomposition of ammonium nitrate. J Chem Phys. 1962;37(8):1583–6.
Li XR, Koseki H. Study on the contamination of chlorides in ammonium nitrate. Process Saf Environ Prot. 2005;83(1):31–7.
Medard LA. Accidental explosions: Types of explosive substances, vol. 2. New York: Wiley; 1989.
Rubtsov YI, Strizhevskii II, Kazakov AI, Moshkovich EB, Andrienko LP. Kinetics of the influence of Cl On thermal-decomposition of ammonium nitrate. J Appl Chem USSR. 1989;62(11):2245–50.
Keenan AG. Differential thermal analysis of the thermal decomposition of ammonium nitrate. J Am Chem Soc. 1955;77(5):1379–80.
Tramm H, Velde H. On the spontaneous decomposition of AN melts. Angew Chem. 1934;47(24):782–3.
Maschio G, Ferrara I, Bassani C, Nieman H. An integrated calorimetric approach for the scale-up of polymerization reactors. Chem Eng Sci. 1999;54(15–16):3273–82.
Richard MN, Dahn JR. Accelerating rate calorimetry study on the thermal stability of lithium intercalated graphite in electrolyte. I. Experimental. J Electrochem Soc. 1999;146(6):2068–77.
Colvin CI, Keenan AG, Hunt JB. Isotopic tracer study of the chloride-catalyzed thermal decomposition of ammonium nitrate. J Chem Phys. 1963;38(12):3033–5.
Acknowledgements
We thank Prof. Liu Dabin for valuable advice and assistance in carrying out the experimental work. This study was supported by the National Natural Science Foundation of China, Grant No. 51174120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, L., Liu, D., Wu, Q. et al. Effect of potassium chloride on thermal stability of ammonium nitrate under acidic conditions. J Therm Anal Calorim 131, 2719–2728 (2018). https://doi.org/10.1007/s10973-017-6748-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6748-8