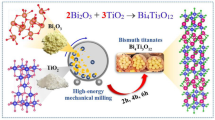
Abstract
The mechanism of Bi4Ti3O12 formation starting from the two constituent oxides has been studied. Starting from a physical mixture, a solid-state reaction occurs between the two oxides that leads at ≈700 °C to a mixture of the two ternary oxides Bi12TiO20 + Bi4Ti3O12 along with unreacted precursor oxides. At T ≈ 830 °C Bi12TiO20 reacts with TiO2 forming Bi4Ti3O12 and, finally, at T ≈ 850 °C, the residual Bi12TiO20 undergoes the peritectic reaction that produces Bi4Ti3O12 plus a liquid phase. However, the formation of Bi4Ti3O12 is not complete at temperatures as high as 900 °C. Starting from a mechanically activated mixture, the intermediate Bi12TiO20 only forms as a minority phase at a lower temperature (T ≈ 550 °C), and then it rapidly reacts to give Bi4Ti3O12. No trace of the peritectic reaction is found in the case of the activated mixture. The complete formation of Bi4Ti3O12 can be obtained by 3-h annealing of the activated mixture at T ≥ 650 °C. The heat capacity of the product phase Bi4Ti3O12 has also been measured in the temperature range 50–300 °C.






Similar content being viewed by others
References
Simoes AZ, Riccardi CS, Cavalcante LS, Gonzalez AHM, Longo E, Varela JA. Size effects of polycrystalline lanthanum modified Bi4Ti3O12 thin film. Mater Res Bull. 2008;43:158–67.
Patwardhan JS, Rahaman MN. Compositional effects on densification and microstructural evolution of bismuth titanate. J Mater Sci. 2004;39:133–9.
Zhang F, Karachi T, Adachi M. Coprecipitation synthesis of nanosized Bi4Ti3O12 particles. Jpn J Appl Phys. 2006;45(9B):7385–8.
Machedo ZS, Ferrari CR, Hernandes AC. Self-propagating high-temperature synthesis of bismuth titanate. Powder Technol. 2004;139:175–9.
Pookmanee P, Uriwilast P, Panichphant S. Hydrothermal synthesis of fine bismuth titanate powders. Ceram Int. 2004;30:1913–5.
Pookmanee P. Chemical synthesis and characterization of lead-free bismuth titanate (Bi4Ti3O12) from the oxalate method. J Ceram Process Res. 2008;9:30–3.
Simoes AZ, Quinelato C, Ries A, Stojanovic BD, Longo E, Varela JA. Preparation of lanthanum doped Bi4Ti3O12 ceramics by the polymeric precursor method. Mater Chem Phys. 2006;98:481–5.
Kojima S, Hushur A, Jiang F, Hamazaki S, Takashige T, Jang MS, Shimada S. Crystallization of amorphous bismuth titanate. J Non-Cryst Solids. 2001;293:250–4.
Morrison AD. Some properties of Bi12TiO20 and the system Bi2O3–TiO2. Ferrolectrics. 1971;2:59–62.
Burton TM. Study of the liquidus in the system Bi2O3–TiO2. J Solid State Chem. 1974;9:173–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berbenni, V., Milanese, C., Bruni, G. et al. Synthesis of Bi4Ti3O12 by high energy milling of Bi2O3–TiO2 (anatase) mixtures. J Therm Anal Calorim 126, 1507–1511 (2016). https://doi.org/10.1007/s10973-016-5807-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5807-x