Abstract
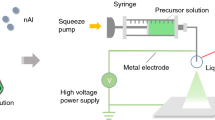
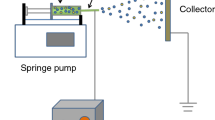
Polyvinylidene fluoride (PVDF) is a potential oxidizer for aluminum powders as well as an excellent binder. The strong electronegative fluorine within the polymer can react with the alumina passivation shell surrounding an aluminum (Al) particle to promote Al particle reactivity. In this study, nanoaluminum (n-Al)/PVDF microsphere particles with diameters of between 1 and 5 μm were successfully prepared with PVDF content of 5, 10 and 15 % by mass using the electrospray deposition method. Thermogravimetric–differential scanning calorimetric analysis shows more intense heat release process (the sharper exothermic peak) compared to n-Al. The combustion properties tested in open air show that all samples can be ignited, while the n-Al/PVDF demonstrated more active reactivity in comparison with n-Al. The burning duration decreases from 3.51 s to 219 ms as the PVDF concentration increases. The work, which shows that using electrospray deposition fabricates the spherical particles consisting of n-Al and reactive binder, expects an approach to the promising n-Al in energetic materials.







Similar content being viewed by others
References
Zhang BY, Huang C, Yan S, Li YC, Cheng Y. Enhanced reactivity of boron, through adding nano-aluminum and wet ball milling. Appl Surf Sci. 2013;286:91–8.
Dreizin EL. Metal-based reactive nanomaterials. Energy Combust. Sci. 2009;35(2):141–67.
Yen NH, Wang LY. Reactive metals in explosives. Propellants Explos Pyrotech. 2012;37(2):143–55.
Xing Xiaoling, Zhao Shengxiang, Huang Wenming, et al. Thermal decomposition behavior of hexanitrohexaazaisowurtzitane and its blending with BTATz (expand) and Al by microcalorimetry. J Therm Anal Calorim. 2015;120:1393–7.
Fathollahi Manoochehr, Behnejad Hassan. A comparative study of thermal behaviors and kinetics analysis of the pyrotechnic compositions containing Mg and Al. J Therm Anal Calorim. 2015;120:1483–92.
Wang LL, Munir ZA, Maximov YM. Thermite reactions: their utilization in the synthesis and processing of materials. J Mater Sci. 1993;28(14):3693–708.
Shimizu A, Saitou J, Hao YJ. Effect of contact points between particles on the reaction rate in the Fe2O3/V2O5 system. J Solid State Ion. 1990;38(3–4):261–9.
Granier JJ, Pantoya ML. Combustion behavior of highly energetic thermites: nano versus micron composites. Propellants Explos Pyrotech. 2005;30(1):53–62.
Severac F, Alphonse P, Esteve A, Bancaud A, Rossi C. High-energy Al/CuO nanocomposites obtained by DNA-directed assembly. Adv Funct Mater. 2012;22(2):323–9.
Brege J, Hamilton C, Crouse C, Barron A. Ultrasmall copper nanoparticles from a hydrophobically immobilized surfactant template. Nano Lett. 2009;9(6):2239–42.
Liakos IL, McAlpine E, Chen XY, Newman R, Alexander MR. Assembly of octadecyl phosphonic acid on the α-Al2O3 (0001) surface of air annealed alumina: evidence for termination dependent adsorption. Appl Surf Sci. 2008;255(5):3276–82.
Oberg K, Persson P, Shchukarev A, Eliasson B. Comparison of monolayer films of stearic acid and methyl stearate on an Al2O3 surface. Thin Solid Films. 2001;397(1–2):102–8.
Karaman ME, Antelmi DA, Pashley RM. The production of stable hydrophobic surfaces by the adsorption of hydrocarbon and fluorocarbon carboxylic acids onto alumina substrates. Colloids Surf A. 2001;182(1–3):285–98.
Crouse C, Pierce C, Spowart J. Influencing solvent miscibility and aqueous stability of aluminum nanoparticles through surface functionalization with acrylic monomers. ACS Appl Mater Interfaces. 2010;2(9):2560–9.
Jouet RJ, Warren A, Rosenberg DM, Bellito VJ, Park K, Zachariah MR. Surface passivation of bare aluminum nanoparticles using perfluoroalkyl carboxylic acids. Chem Mater. 2005;17(11):2987–96.
Kappagantula KS, Farley C, Pantoya ML, Horn J. Tuning energetic material reactivity using surface functionalization of aluminum fuel. J Phys Chem C. 2012;116(46):24469–75.
Ayoman E, Hosseini SG. Synthesis of CuO nanopowders by high-energy ball-milling method and investigation of their catalytic activity on thermal decomposition of ammonium perchlorate particles. J Therm Anal Calorim. 2016;123:1213–24.
Umbrajkar SM, Seshadri S, Schoenitz M, Hoffmann VK, Dreizin EL. Aluminum-rich Al-MoO3 nanocomposite powders prepared by arrested reactive milling. J Propul Power. 2008;24(2):192–8.
Umbrajkar SM, Trunov MA, Schoenitz M, Dreizin EL, Broad R. Arrested reactive milling synthesis and characterization of sodium-nitrate based reactive composites. Propellants Explos Pyrotech. 2007;32(1):32–41.
Sippel TR, Son SF, Groven LJ. Altering reactivity of aluminum with selective inclusion of polytetrafluoroethylene through mechanical activation. Propellants Explos Pyrotech. 2013;289(2):286–95.
Sippel TR, Son SF, Groven LJ. Modifying aluminum reactivity with poly(carbon monofluoride) via mechanical activation. Propellants Explos Pyrotech. 2013;38(3):321–6.
Yan S, Jian GQ, Zachariah MR. Electrospun nanofiber-based thermite textiles and their reactive properties. ACS Appl Mater Interfaces. 2012;4(12):6432–5.
Li Xiangyu, Huang Chuan, Yang Hongtao, Li Yanchun, Cheng Yi. Thermal reaction properties of aluminum/copper (II) oxide/poly(vinylidene fluoride) nanocomposite. J Therm Anal Calorim. 2016;124:899–907.
Correia DM, Gonçalves R, Ribeiro C, Lanceros-Mendez S, et al. Electrosprayed poly(vinylidene fluoride) microparticles for tissue engineering applications. RSC Adv. 2014;4:33013–21.
Gonçalves R, Martins P, Correia DM, et al. Development of magnetoelectric CoFe2O4/poly(vinylidene fluoride) microspheres. RSC Adv. 2015;5:35852–7.
Li X, Guerieri P, Zhou W, Huang C, Zachariah MR. Laminate nano-composite with enhanced propellant properties. ACS Appl Mater Interfaces. 2015;17(7):9103–9.
Huang C, Yang HT, Li YC, Cheng Y. Characterization of aluminum/poly(vinylidene fluoride) by thermogravimetric analysis, differential scanning calorimetry, and mass spectrometry. Anal Lett. 2015;48(13):2011–21.
Almería Begoña, Gomez Alessandro. Electrospray synthesis of monodisperse polymer particles in a broad (60 nm–2 µm) diameter range: guiding principles and formulation recipes. J Colloid Interface Sci. 2014;417:121–30.
Juknelevicius Dominykas, Kubilius Rytis, Ramanavicius Arunas. Oxidizer ratio and oxygen balance influence on the emission spectra of green-colored pyrotechnic flames. Eur J Inorg Chem. 2015;33:5511–5.
Botelho G, Lanceros-Mendez S, Goncalves AM, Sencadas V, Rocha JG. Relationship between processing conditions, defects and thermal degradation of poly(vinylidene fluoride) in the β-phase. J Non-Cryst Solids. 2008;354(1):72–8.
Wang Haiyang, Jian Guoqiang, Yan Shi, DeLisio Jeffery B, Huang Chuan, Zachariah Michael R. Electrospray formation of gelled nano-aluminum microspheres with superior reactivity. Appl Mater Interfaces. 2013;5:6797–801.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Huang, C. & Chen, H. Tuning reactivity of nanoaluminum with fluoropolymer via electrospray deposition. J Therm Anal Calorim 127, 2293–2299 (2017). https://doi.org/10.1007/s10973-016-5801-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5801-3