Abstract
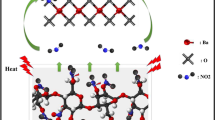
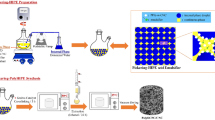
The microencapsulation of ammonium azide particles (NH4N3) with fibrous nitrocellulose has been carried out through a solvent/non-solvent procedure which is based on the coacervation principle. X-ray diffraction, scanning electron microscope, Fourier transform infrared spectroscopy, thermogravimetry–differential thermal analysis and differential scanning calorimetry techniques were used to characterize the structure, morphology and thermal stability of treated ammonium azide particles. Taguchi L-9 orthogonal array design method was exploited for assessment of effectiveness of experimental parameters on the coating properties. The investigated parameters were percent of coating agent, addition time of non-solvent, volume of non-solvent and stirring speed of the mixture (revolutions per minute, rpm). The individual effect of each parameter on the thermal stability of NH4N3 particle, determined by thermal analysis methods, was quantitatively evaluated by the analysis of variance. The statistical results revealed that the most stabilized coated NH4N3 particles can be obtained by using 2 % (w/w) of nitrocellulose as coating agent and by adding of 70 mL n-hexane as non-solvent within 50 min under stirring at 90 rpm, where the sublimation temperature of treated sample increases about 60 °C with respect to uncoated one and reaches to 186.5 °C. Also, the kinetic parameters of the sublimation processes of pure and microencapsulated NH4N3 particles that stabilized at this condition were obtained from the differential scanning calorimetry data by non-isothermal methods proposed by ASTM E696. Finally, the results of this study illustrated that the efficiency of the proposed chemometric method is higher than that of sequential experimental technique.





Similar content being viewed by others
References
Ng WL, Field JE. Sublimation of ammonium azide by differential scanning calorimetric and thermogravimetric. Thermochim Acta. 1985;84:133–40.
Prince E, Choi CS. Ammonium azide. Acta Crystallogr B. 1978;34:2606–8.
Ledgard JB. The preparatory manual of explosives, 2nd ed., ISBN: 0-9727863-O-9, Copyright @ 2003 by The Paranoid Publications Group; 2003.
Ledgard J, The preparatory manual of black powder and pyrotechnics, version 1.4, ISBN: 9780615174273, Jared Ledgard; 2007.
Meyer R, Kohler J, Homburg A. Explosives. 5th ed. Essen: Wiley-VCH; 2002.
Amorim HS, Amaral JMR, Pattison P, Ludka IP, Mendes JC. Ammonium azide: a commented example of an Ab initio structure (Re-) determination from X-ray powder diffraction. Rev Soc Quím Méx. 2002;46:313–9.
Ball DW. High-level ab initio calculations on hydrogen–nitrogen compounds. thermochemistry of tetrazetidine, N4H4. J Mol Struct Theochem. 2002;619:37–43.
Crowhurst JC, Zaug JM, Radousky HB, Steele BA, Landerville AC, Oleynik II. Ammonium azide under high pressure: a combined theoretical and experimental study. J Phys Chem A. 2014;118:8695–700.
Landerville AC, Steele BA, Oleynik II. Ammonium azide under hydrostatic compression. J Phys Conf Ser. 2014;500:162006/1-6.
Liu Q-J, Zeng W, Liu F-S, Liu Z-T. First-principles study of hydronitrogen compounds: molecular crystalline NH4N3 and N2H5N3. Comput Theor Chem. 2013;1014:37–42.
Yedukondalu N, Ghule VD, Vaitheeswaran G. Computational study of structural, electronic and optical properties of crystalline NH4N3. J Phys Chem C. 2012;116:16910–7.
Wu X, Cui H, Zhang J, Cong R, Zhu H, Cui Q. High pressure synchrotron X-ray diffraction and Raman scattering studies of ammonium azide. Appl Phys Lett. 2013;102:121902/1-4.
Dows DA, Whittle E, Pimentel GC. Infrared spectrum of solid ammonium azide: a vibrational assignment. J Chem Phys. 1955;23:1475–9.
Boutin H, Trevino S, Prask HJ. Low-frequency molecular motions in ammonium azide. J Chem Phys. 1966;45:401–2.
Medvedev SA, Eremets MI, Evers J, Klapoetke TM, Palasyuk T, Trojan IA. Pressure induced polymorphism in ammonium azide (NH4N3). Chem Phys. 2011;386:41–4.
Wu X, Ma F, Ma C, Cui H, Liu Z, Zhu H, Wang X, Cui Q. Pressure-driven variations of hydrogen bonding energy in ammonium azide (NH4N3): IR absorption and Raman scattering studies. J Chem Phys. 2014;141:024703/1-8.
Medvedev SA, Palasyuk T, Trojan IA, Naumov PG, Evers J, Klapötke TM, Eremets MI. Pressure-tuned vibrational resonance coupling of intramolecular fundamentals in ammonium azide (NH4N3). Vib Spectrosc. 2012;58:188–92.
Iqbal Z, Malhotra ML. Vibrational spectrum of crystalline ammonium azide. Spectrochim Acta Part A. 1971;27:441–6.
Yakovleva GS, Kurbangalina RK, Stesik LN. Detonation properties of ammonium azide. Combust Explos Shock Waves. 1997;13:405–7.
Farhat K, Batonneau Y, Florea O, Kappenstein C. Preparation and use of ammonium azide as a fuel additive to ionic oxidizer solutions, physicochemical properties, thermal and catalytic decomposition. In: 42nd AIAA/ASME/SAE/ASEE, joint propulsion conference, 9–12 July 2006. California: Sacramento; 2006.
Donald H, Jenkins B. Viscosity B-coefficient of ammonium azide and the enthalpy of formation of the azide anion. J Solution Chem. 1993;22:1029–31.
Eslami A, Hosseini SG, Shariaty SHM. Stabilization of ammonium azide particles through its microencapsulation with some organic coating agents. Powder Technol. 2011;208:137–43.
Finch CA. Microencapsulation, Ulmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH Verlag GmbH; 2002.
Aghbashlo M, Mobli H, Rafiee S, Madadlou A. Optimization of emulsification procedure for mutual maximizing the encapsulation and exergy efficiencies of fish oil microencapsulation. Powder Technol. 2012;225:107–17.
Kha TC, Nguyen MH, Roach PD, Stathopoulos CE. Microencapsulation of Gac oil: optimisation of spray drying conditions using response surface methodology. Powder Technol. 2014;264:298–309.
Kwon YS, Gromov AA, Strokova JI. Passivation of the surface of aluminum nanopowders by protective coatings of the different chemical origin. Appl Surf Sci. 2007;253:5558–64.
Gromov A, Ilyin A. Characterization of aluminum powders: II. Aluminum nanopowders passivated by non-inert coatings. Propellant Explos Pyrotech. 2006;31:401–8.
Zhang L, Ranade MB, Gentry JW. Formation of organic coating on ultrafine silver particles using a gas-phase process. J Aerosol Sci. 2004;35:457–71.
Herbig JA. Encyclopedia of chemical technology (Kirk-Othmer), vol. 13. New York: Interscience; 2003.
Chen X, Jiao C, Zhang J. Microencapsulation of ammonium polyphosphate with hydroxyl silicone oil and its flame retardance in thermoplastic polyurethane. J Therm Anal Calorim. 2011;104:1037–43.
Wang N, Mi L, Wu Y, Zhang J, Fang Q. Double-layered co-microencapsulated ammonium polyphosphate and mesoporous MCM-41 in intumescent flame-retardant natural rubber composites. J Therm Anal Calorim. 2014;115:1173–81.
Eslami A, Hosseini SG, Asadi V. The effect of microencapsulation with nitrocellulose on thermal properties of sodium azide particles. Prog Org Coat. 2009;65:269–74.
Eslami A, Hosseini SG, Bazrgary M. Improvement of thermal decomposition properties of ammonium perchlorate particles using some polymer coating agents. J Therm Anal Calorim. 2013;113:721–30.
Roy RK. A primer on the Taguchi method. New York: Van Nostrand Reinhold; 1990.
Taguchi G. Systems of experimental design. Kraus: New York, Vol. 1, 2; 1987.
Ross PJ. Taguchi techniques for quality engineering. New York: Mc Graw-Hill; 1988.
Mishra N, Patra N, Pandey S, Salerno M, Sharon M, Sharon M. Taguchi method optimization of wax production from pyrolysis of waste polypropylene. J Therm Anal Calorim. 2014;117:885–92.
Lai Y, Huang H, Huang Q, Zhang H, Guo Z. Optimization of the experimental conditions for the synthesis of micro-size monodisperse spherical silver powders using Box–Behnken design. Powder Technol. 2014;263:7–13.
Hosseini SG, Eslami A. Orthogonal array design method for optimization experiments of sodium azide microencapsulation with stearic acid. Prog Org Coat. 2010;68:313–8.
Hosseini SG, Pourmortazavi SM, Fathollahi M. Orthogonal array design for the optimization of silver recovery from waste photographic paper. Sep Sci Technol. 2004;8:1953–65.
Chen G, Chen J, Li J, Guo S, Srinivasakannan C, Peng J. Optimization of combined microwave pretreatment–magnetic separation parameters of ilmenite using response surface methodology. Powder Technol. 2012;232:58–63.
Dargahi M, Kazemian H, Soltanieh M, Hosseinpour M, Rohani S. High temperature synthesis of SAPO-34: applying an L9 Taguchi orthogonal design to investigate the effects of experimental parameters. Powder Technol. 2012;217:223–30.
Hou T-H, Su C-H, Liu W-L. Parameters optimization of a nano-particle wet milling process using the Taguchi method, response surface method and genetic algorithm. Powder Technol. 2007;173:153–62.
Hanley KJ, O’Sullivan C, Oliveira JC, Cronin K, Byrne EP. Application of Taguchi methods to DEM calibration of bonded agglomerates. Powder Technol. 2011;210:230–40.
Heng D, Lee SH, Kwek JW, Ng WK, Chan H-K, Tan RBH. Assessing the combinatorial influence of climate, formulation and device on powder aerosolization using the Taguchi experimental design. Powder Technol. 2012;226:253–60.
Edrissi M, Samadanian-Isfahani SA, Soleymani M. Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of reactive black 8 dye. Powder Technol. 2013;249:378–85.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Hosseini SG. Orthogonal array design for the optimization of supercritical carbon dioxide extraction of different metals from a solid matrix with cyanex 301 as a ligand. J Chem Eng Data. 2004;49:1530–4.
Frierson WJ. Inorganic syntheses, vol. 2. New York: McGraw-Hill Book Company, Inc; 1946.
Tarasov NB, Petrov OE, Faizullin DA, Davydov MN. FTIR-spectroscopic studies of the fine structure of nitrocellulose treated by Desulfovibrio desulfuricans. Anaerobe. 2005;11:312–4.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. B Chem Soc Jpn. 1965;38:1881–6.
ASTM E698-05. Standard test method for Arrhenius kinetic constants for thermally unstable materials. 2001. doi:10.1520/E0698-05.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141–4.
Hosseini SG, Eslami A. Investigation on the reaction of powdered tin as a metallic fuel with some pyrotechnic oxidizers. Propellant Explos Pyrotech. 2011;36:175–81.
Eslami A, Hosseini SG, Improving safety performance of lactose-fueled binary pyrotechnic systems of smoke dyes. J Therm Anal Calorim. 104:671-678. 2011. http://www.springerlink.com/content/1388-6150/104/2/.
Eslami A, Hosseini SG, Pourmortazavi SM. Thermoanalytical investigation on some boron-fuelled binary pyrotechnic systems. Fuel. 2008;87:3339–43.
Hosseini SG, Eslami A. Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems. J Therm Anal Calorim. 2010;101:1111–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.G., Eslami, A. The exploration of the influence of microencapsulation processing parameters on the stabilization of ammonium azide particles. J Therm Anal Calorim 126, 455–466 (2016). https://doi.org/10.1007/s10973-016-5540-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5540-5