Abstract
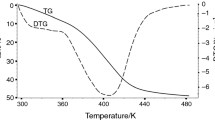
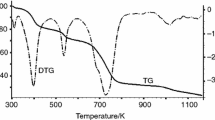
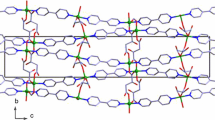
Metal–organic frameworks (MOFs) have promising practical applications in gas storage, in separation and purification of substances, and in catalysis. The standard process of the MOF production begins from the synthesis of the inclusion compound; the molecules of the used organic solvent are caught in the channels and caves of the MOF structure. These primary included guest molecules are excluded further by the evacuation or by the heating. We studied the correlation between the thermal (kinetic) stability of the inclusion compounds and the framework and guest molecules properties. The thermogravimetric curves were used for the kinetic studies; kinetic parameters of decomposition were estimated within the approaches of the non-isothermal kinetics (“model-free” kinetics and nonlinear regression methods). Studied compounds series: [Zn2(bdc)2(dabco)]·4DMF and [Zn4(DMF)(ur)2(ndc)4]·5DMF, [Zn4(DMF)(ur)2(ndc)4]·6benzene and [Zn4(DMF)(ur)2(ndc)4]·5toluene (bdc = bdc2− = terephthalate, dabco = 1,4-diazabicyclo[2.2.2]octane, DMF = dimethylformamide; ur = hexamethylenetetramine, ndc2− = 2,6-naphthalenedicarboxylate); [Mn(HCOO)2]·0.33dioxane and [Li2(H2btc)]·dioxane (H4btc = 1,2,4,5-benzenetetracarboxylic acid). The values of commonly used molecular kinetic diameters do not take into consideration the real molecular form and the size (e.g. benzene and toluene molecules have the same kinetic diameters 5.85 Å). Therefore, the ease of removal of guest molecules does not correlate with them directly.








Similar content being viewed by others
References
Hosny NM. Solvothermal synthesis, thermal and adsorption properties of metal–organic frameworks Zn and CoZn (DPB). J Therm Anal Calorim. 2015;122:89–95.
Kirillov AM. Hexamethylenetetramine: an old new building block for design of coordination polymers. Coord Chem Rev. 2011;255:1603–22.
Mani-Biswas M, Cagin T. Insights from theoretical calculations on structure, dynamics, phase behavior and hydrogen sorption in nanoporous metal organic frameworks. Comput Theor Chem. 2012;987:42–56.
Lyszczek R, Iwan M. Investigation of desolvation process in lanthanide dinicotinates. J Therm Anal Calorim. 2011;103:633–9.
Jiang C-H, Song L-F, Jiao C-L, Zhang J, Sun L-X, Xu F, Du Y, Cao Z. Exceptional thermal stability and thermodynamic properties of lithium based metal–organic framework. J Therm Anal Calorim. 2011;103:373–80.
Sha J-Q, Wu L-H, Li S-X, Yang X-N, Zhang Yu, Zhang Q-N, Zhu P-P. Synthesis and structure of new carbohydrate metal–organic frameworks and inclusion complexes. J Mol Struct. 2015;1101:14–20.
Dybtsev DN, Yutkin MP, Peresypkina EV, Virovets AV, Serre C, Ferey G, Fedin VP. Isoreticular homochiral porous metal–organic structures with tunable pore sizes. Inorg Chem. 2007;46:6843–5.
Zhou Y-X, Sun L-X, Cao Z, Zhang J, Xu F, Song L-F, Zhao Z-M, Zou Y-J. Heat capacities and thermodynamic properties of M(HBTC)(4,4′-bipy)·3DMF (M = Ni and Co). J Therm Anal Calorim. 2012;110:949–54.
Jang X, Jiao C-L, Sun Y-J, Li Z-B, Liu S, Zhang J, Wang Z-Q, Sun L-X, Xu F, Zhou H, Sawada Y. Heat capacities and thermodynamic properties of Co(3,5-PDC)(H2O). J Therm Anal Calorim. 2013;112:1579–85.
Deb N. An investigation on solid-state pyrolytic decomposition of barium trihemiaquatris(oxalato)lanthanate(III)decahydrate using TG–DTA in air and DSC in nitrogen. J Therm Anal Calorim. 2013;112:1415–21.
Yang Q, Xie G, Chen S, Gao S. Synthesis, structure and thermodynamics of supermolecular compound [Ni3(Hdatrz)6(sca)2(H2O)4]sca·11H2O (Hdatrz = 3,5-diamino-1,2,4-triazole, H2sca = succinic acid). J Therm Anal Calorim. 2012;110:979–84.
Logvinenko VA, Yutkin MP, Zavakhina MS, Fedin VP. Porous metal–organic frameworks (MOFs) as matrices for inclusion compounds: kinetic stability under heating. J Therm Anal Calorim. 2012;109:555–60.
Dybtsev DN, Yutkin MP, Samsonenko DG, Fedin VP, Nuzhdin AL, Bezrukov AA, Bryliakov KP, Talsi EP, Belosludov RV, Mizuseki H, Kawazoe Y, Subbotin OS, Belosludov VR. Modular homochiral porous coordination polymers: rational design, enantioselective guest exchange sorption and ab initio calculations of host–guest interactions. Chem Eur J. 2010;10:348–56.
Sapchenko SA, Samsonenko DG, Dybtsev DN, Melgunov MS, Fedin VP. Microporous sensor: gas sorption, guest exchange and guest-dependent luminescence of metal–organic framework. Dalton Trans. 2011;40:2196–203.
Dybtsev DN, Chun H, Kim K. Rigid and flexible: a highly porous metal–organic framework with unusual guest-dependent dynamic behavior. Angew Chem Int Ed. 2004;43:5033–6.
Dybtsev DN, Chun H, Yoon SH, Kim D, Kim K. Microporous manganese formate: a simple metal–organic porous material with high framework stability and highly selective gas sorption properties. J Am Chem Soc. 2004;126:32–3.
Logvinenko V, Dybtsev D, Fedin V, Drebushchak V, Yutkin M. The stability of inclusion compounds under heating. Part I. Inclusion compounds of micro-porous manganese formate with included dioxane Mn(HCOO)2·1/3C4H8O2 and tetra-hydrofurane {Mn(HCOO)2·1/3C4H8O}. J Therm Anal Calorim. 2007;90:463–7.
Logvinenko V. Stability of supramolecular compounds under heating. Thermodynamic and kinetic aspects. J Therm Anal Calorim. 2010;101:577–83.
Aliev SB, Samsonenko DG, Rakhmanova MI, Dybtsev DN, Fedin VP. Syntheses and structural characterization of lithium carboxylate frameworks and guest-dependent photoluminescence study. Cryst Growth Des. 2014;14:4355–63.
Netzsch Thermokinetics. In: Netzsch Thermokinetics. http://www.netzsch-thermal-analysis.com/us/products-%20%20solutions/advanced-software/thermokinetics.html.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J Polym Sci. 1963;6:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203:159–65.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand. 1966;70:478–523.
Opfermann J, Kaisersberger E. An advantageous variant of the Ozawa–Flynn–Wall analysis. Thermochim Acta. 1992;203:167–75.
Opfermann JR, Kaisersberger E, Flammersheim HJ. Model-free analysis of thermo-analytical data-advantages and limitations. Thermochim Acta. 2002;391:119–27.
Vyazovkin S. Model-free kinetics: staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Simon P. Single-step kinetics approximation employing non-Arrhenius temperature functions. J Therm Anal Calorim. 2005;79:703–8.
Simon P. The single-step approximation: attributes, strong and weak sides. J Therm Anal Calorim. 2007;88:709–15.
Borchard HJ, Daniels F. The application of differential thermal analysis to the study of reaction kinetics. J Am Chem Soc. 1957;79:41–6.
Vyazovkin S, Burnham AK, Criado JM, Luis A, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis K, Di Lorenzo M-R, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol J-J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Berlin: Springer; 2015.
Simon P, Thomas P, Dubaj T, Cibulkova Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9.
Simon P, Dubaj T, Cibulkova Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature range of measurement. J Therm Anal Calorim. 2015;120:231–8.
Sestak J. Is the original Kissinger equation obsolete today: not obsolete the entire non-isothermal kinetics? J Therm Anal Calorim. 2014;117:3–7.
Galwey AK. What theoretical and/or chemical significance is to be attached to the magnitude of an activation energy determined for a solid-state decomposition by thermal analysis? J Therm Anal Calorim. 2006;86:267–86.
Roura P, Farjas J. Analytical solution for the Kissinger equation. J Mater Res. 2009;24:3095–8.
Holba P, Šesták J. Imperfections of Kissinger evaluation method and crystallization kinetics. Glass Physics Chem. 2014;40:486–91 (translation from Russian journal).
Dubaj T, Cibulková Z, Šimon P. An incremental isoconversional method for kinetic analysis based on the orthogonal distance regression. J Comput Chem. 2015;36:392–8.
Logvinenko V. Stability and reactivity of coordination and inclusion compounds in the reversible processes of thermal dissociation. Thermochim Acta. 1999;340–341:293–9.
Logvinenko V, Drebushchak V, Pinakov D, Chekhova G. Thermodynamic and kinetic stability of inclusion compounds under heating. J Therm Anal. 2007;90:23–30.
Logvinenko VA, Sapchenko SA, Fedin VP. Thermal decomposition of inclusion compounds on the base of the metal–organic framework [Zn4(dmf)(ur)2(ndc)4]. Part I. J Therm Anal Calorim. 2014;117:747–53.
Solomons TWG, Fryhle CB. Organic Chemistry. 9th ed. New York: Wiley; 2006.
Hauptmann S, Graefe J, Remane H. Lehrbuch der organischen Chemie. Leipzig: VEB Deutscher Verlag fur Grundstoffindustrie; 1976.
Poling BE, Prausnitz JM, O’Connell JP. The properties of gases and liquids. New York: McGraw-Hill; 2001.
Logvinenko VA, Sapchenko SA, Fedin VP. Thermal decomposition of inclusion compounds on the base of the metal–organic framework [Zn4(dmf)(ur)2(ndc)4]. Part II. J Therm Anal Calorim. 2016;123:697–702.
Logvinenko V, Dybtsev D, Fedin V, Drebushchak V, Yutkin M. The stability of inclusion compounds under heating. Part 2. Inclusion compounds of layered zinc camphorate, linked by linear N-donor ligands. J Therm Anal Calorim. 2010;100:183–93.
Serin B, Ellickson RT. Determination of diffusion coefficients. J Chem Phys. 1941;9:742–7.
Logvinenko VA, Aliev SB, Fedin VP. Thermal (kinetic) stability of the inclusion compound on the base of Li-containing MOF [Li2(H2btc)]·dioxane. J Therm Anal Calorim. 2015;120:53–8.
Breck DW. Zeolite molecular sieves: structure, chemistry and use. New York: Wiley; 1974.
Matteucci S, Yampolskii Y, Freeman BD, Pinnau I. Transport of gases and vapors in glassy and rubbery polymers. In: Freeman BD, Yampolskii Y, Pinnau I, editors. Materials science of membranes for gas and vapor separation. New York: Wiley; 2006. p. 1–47.
Elshof JE, Abadal CR, Sekulic J, Chowdhury SR, Blank DHA. Transport mechanisms of water and organic solvents through microporous silica in pervaporization of binary liquids. Microporous Mesoporous Mater. 2003;65:197–208.
Freude D. Molecular physics. Letter notes. Chapter 2. Size, mass and kinetics of molecules. Version October 2004. http://www.uni-leipzig.de/~energy/pdf/freume2.pdf.
Mehio N, Dai Sh, Jiang D. Quantum mechanical basis for kinetic diameters of small gaseous molecules. J Phys Chem A. 2014;118:1150–4.
Acknowledgements
This work was partially supported by the Russian Foundation for Basic Research (Grant 14-03-00291).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logvinenko, V.A., Aliev, S.B., Bolotov, V.A. et al. Thermal (kinetic) stability of inclusion compounds on the basis of porous metal–organic frameworks. J Therm Anal Calorim 127, 779–787 (2017). https://doi.org/10.1007/s10973-016-5398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5398-6