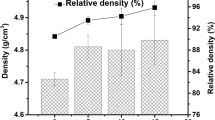
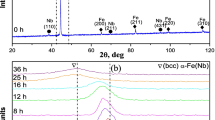
Abstract
In this study, mechanical activation process was used for intimate mixing as well as producing finely ground particles, increased surface area and improved chemical reactivity of milled materials for producing SrTiO3 from commercially pure strontium carbonate and TiO2 as a contributive process. Characterization of milled powder mixture by X-ray diffraction analysis showed that disappearing, decreasing and/or shifting of the patterns occurred with mechanical activation that means amorphization was taken place. Amorphization was also demonstrated by FT-IR analysis where shift of band centers as well as the decrement of transmittance related to CO3 was observed. Advantage of amorphization was established with high-temperature XRD analysis which showed 1300 °C was not enough for non-activated mixture to form SrTiO3, whereas structure only composed of SrTiO3 at 1000 °C for activated ones. The reason for this phenomenon was investigated by DTA-TG analysis, and it was based on energy accumulation originated from mechanical activation that corresponds to peak temperature shifting to the lower temperatures and CO2 liberation at mechanical activation step arising from local temperature rising at the vial during high-energy milling that was understood from peak temperature, and area decrement of endothermic peak corresponds to decomposition of SrCO3.








Similar content being viewed by others
References
Psiuk B, Szade J, Wrzalik R, Osadnik M, Wala T. Milling-induced phenomena in SrTiO3. Ceram Int. 2014;40(5):6957–61.
Zhang J, Huang M, Yanagisawa K, Yao S. NaCl–H2O-assisted preparation of SrTiO3 nanoparticles by solid state reaction at low temperature. Ceram Int. 2015;41(4):5439–44.
Zhang Y, Zhong L, Duan D. Single-step hydrothermal synthesis of strontium titanate nanoparticles from crystalline anatase titanium dioxide. Ceram Int. 2015;41(10):13516–24.
Shih SJ, Tzeng WL. Manipulation of morphology of strontium titanate particles by spray pyrolysis. Powder Technol. 2014;264:291–7.
Stanulis A, Selskis A, Ramanauskas R, Beganskiene A, Kareiva A. Low temperature synthesis and characterization of strontium stannate–titanate ceramics. Mater Chem Phys. 2011;130(3):1246–50.
Berbenni V, Marini A, Bruni G. Effect of mechanical activation on the preparation of SrTiO3 and Sr2TiO4 ceramics from the solid state system SrCO3–TiO2. J Alloy Compd. 2001;329(1–2):230–8.
Hao Y, Wang X, Li L. Highly dispersed SrTiO3 nanocubes from a rapid sol-precipitation method. Nanoscale. 2014;6:7940–6.
Zhang SY, Lin YH, Nan CW, Zhao R, He J. Magnetic and electrical properties of (Mn, La)-codoped SrTiO3 thin films. J Am Ceram Soc. 2008;91(10):3263–6.
Obut A, Baláž P, Girgin İ. Direct mechanochemical conversion of celestite to SrCO3. Miner Eng. 2006;19(11):1185–90.
Davar F, Salavati-Niasari M, Baskoutas S. Temperature controlled synthesis of SrCO3 nanorods via a facile solid-state decomposition rout starting from a novel inorganic precursor. Appl Surf Sci. 2011;257(9):3872–7.
Tipcompor N, Thongtem T, Phuruangrat A, Thongtem S. Characterization of SrCO3 and BaCO3 nanoparticles synthesized by cyclic microwave radiation. Mater Lett. 2012;87:153–6.
Zhang DL. Processing of advanced materials using high-energy mechanical milling. Prog Mater Sci. 2004;49(3–4):537–60.
Layek S, Verma HC, Garg A. Enhancement in magnetic properties of Ba-doped BiFeO3 ceramics by mechanical activation. J Alloy Compd. 2015;651:294–301.
Ashrafi H, Emadi R, Foroushani RZ. Synthesis and characterization of mullite–zirconia nanostructured composite by combined mechanical activation and reaction sintering. Adv Powder Technol. 2015;26(5):1452–7.
Li XH, Zhang YJ, Pan LP, Wei YS. Effect of mechanical activation on dissolution kinetics of neutral leach residue of zinc calcine in sulphuric acid. Trans Nonferrous Met Soc China. 2013;23(5):1512–9.
Erdemoğlu M, Aydoğan S, Gock E. Effects of intensive grinding on the dissolution of celestite in acidic chloride medium. Miner Eng. 2009;22(1):14–24.
Baláž P, Achimovičová M, Baláž M, Billik P, Cherkezova-Zheleva Z, Criado JM, Delogu F, Dutková E, Gaffet E, Gotor FJ, Kumar R, Mitov I, Rojac T, Senna M, Streletskii A, Wieczorek-Ciurowa K. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev. 2013;42:7571–637.
Tromans D, Meech JA. Enhanced dissolution of minerals: stored energy, amorphism and mechanical activation. Miner Eng. 2001;14(11):1359–77.
Ungár T. Microstructural parameters from X-ray diffraction peak broadening. Scripta Mater. 2004;51:777–81.
Ahmadzadeh M, Ataie A, Mostafavi E. The effects of mechanical activation energy on the solid-state synthesis process of BiFeO3. J Alloy Compd. 2015;622:548–56.
López MCB, Fourlaris G, Rand B, Riley FL. Characterization of barium titanate powders: barium carbonate identification. J Am Ceram Soc. 1999;82(7):1777–86.
Tunç T, Apaydın F, Yıldız K. Effects of mechanical activation on the structure of nickeliferous laterite. Acta Phys Pol A. 2013;123(2):349–51.
Tunç T, Apaydin F, Yildiz K. Structural alterations and thermal behaviour of mechanically activated alunite ore. J Therm Anal Calorim. 2014;118:883–9.
Baláž P. Mechanochemistry in nanoscience and minerals engineering. Berlin: Springer; 2008.
Ptáček P, Bartoníčková E, Švec J, Opravil T, Šoukal F, Frajkorová F. The kinetics and mechanism of thermal decomposition of SrCO3 polymorphs. Ceram Int. 2015;41(1):115–26.
Chen YH, Huang YC, Jiang WD. Study on thermal properties of nanocrystalline strontianite. J Non-Cryst Solids. 2010;356(28–30):1530–2.
Robbins SA, Rupard RG, Weddle BJ, Maull TR, Gallagher PK. Some observations on the use of strontium carbonate as a temperature standard for DTA. Thermochim Acta. 1995;269–270:43–9.
Lander JJ. Polymorphism and anion rotational disorder in the alkaline earth carbonates. J Chem Phys. 1949;17(10):892–901.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunç Parlak, T., Apaydin, F. & Yildiz, K. Formation of SrTiO3 in mechanically activated SrCO3–TiO2 system. J Therm Anal Calorim 127, 63–69 (2017). https://doi.org/10.1007/s10973-016-5385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5385-y