Abstract
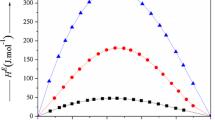
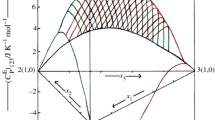
The excess molar enthalpies, \( H_{{\text{123}}}^{\text{E}} \), data of ternary 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + pyrrolidin-2-one or 1-methyl pyrrolidin-2-one (2) + cyclopentanone or cyclohexanone (3) mixtures have been measured at 298.15 K and 0.1 M Pa using microdifferential scanning calorimeter. The \( H_{{\text{123}}}^{\text{E}} \) values of [emim][BF4] (1) + 2-Py (2) + cyclopentanone or cyclohexanone (3) are endothermic, while those for [emim][BF4] (1) + NMP (2) + cyclopentanone or cyclohexanone (3) mixtures are negative over entire composition of x 1 and x 2. The observed data of \( H_{{\text{123}}}^{\text{E}} \) have been investigated in terms of Graph and Prigogine–Flory–Patterson theories. The results indicate that \( H_{{\text{123}}}^{\text{E}} \) values estimated by Graph theory compare well with experimental values.




Similar content being viewed by others
References
Xu H, Zhao D, Xu P, Liu F, Gao G. Conductivity and viscosity of 1-allyl-3-methyl-imidazolium chloride + water and + ethanol from 293.15 K to 333.15 K. J Chem Eng Data. 2005;50:133–5.
Zhang S, Li X, Chen H, Wang J, Zhang J, Zhang M. Determination of physical properties for the binary system of 1-ethyl-3-methylimidazolium tetrafluoroborate + H2O. J Chem Eng Data. 2004;49:760–4.
Arce A, Soto A, Ortega J, Sabater G. Viscosities and volumetric properties of binary and ternary mixtures of tris(2-hydroxyethyl) methylammonium methylsulfate + water + ethanol at 298.15 K. J Chem Eng Data. 2008;53:770–5.
Abdulagatov IM, Tekin A, Safarov J, Shahverdiyev A, Hassel E. High-pressure densities and derived volumetric properties (excess, apparent and partial molar volumes) of binary mixtures of methanol + [BMIM][PF6]. J Solut Chem. 2008;37:801–33.
Saravanan R, Maiya MP. Comparison of methanol-based working fluid combinations for a bubble pump-operated vapour absorption refrigerator. Int J Energy Res. 1998;22:715–31.
Gonzalez B, Calvar N, Gomez E, Dominguez A. Physical properties of the ternary system (ethanol + water + 1-butyl-3-methylimidazolium methylsulphate) and its binary mixtures at several temperatures. J Chem Thermodyn. 2008;40:1274–81.
Guttman TK, Myslinski A, Wilczura HJ. Molar excess enthalpies of binary mixtures of pyridine bases + hexan-1-OL. J Therm Anal Cal. 1984;29:173–7.
Benson GC, Luo B. Lu BCY. Excess enthalpies of dibutyl ether + nK -alkane mixtures at 298.15. Can J Chem. 1988;66:531–4.
Vicente IG, Lisbona NG, Velasco I, Otin S, Embid JM, Kehiaian HV. Excess enthalpies of 1-chloroalkane + benzene. Measurement and analysis in terms of group contributions (DISQUAC). Fluid Phase Equilib. 1989;49:251–62.
Lancaster NM, Wormald CJ. Excess molar enthalpies of nine binary steam mixtures: new and corrected values. J Chem Eng Data. 1990;35:11–6.
Ue M, Takeda M, Takahashi M, Takehara M. Ionic liquids with low melting points and their application to double-layer capacitor electrolytes batteries and energy conversion. Electrochem Solid State Lett. 2002;5:A119–21.
Balducci A, Bardi U, Caporali S, Mastragostino M, Soavi F. Ionic liquids for hybrid supercapacitors. Electrochem Commun. 2004;6:566–70.
Sato T, Masuda G, Takagi K. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim Acta. 2004;49:3603–11.
Wang P, Zakeeruddin SM, Exnar I, Grätzel M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem Commun. 2002;24:2972–3.
Souza RFD, Padilha JC, Goncalves RS, Dupont J. Room temperature dialkylimidazolium ionic liquid-based fuel cells. Electrochem Commun. 2003;5:728–31.
García B, Lavallée S, Perron SG, Michot C. Room temperature molten salts as lithium battery electrolyte. Electrochim Acta. 2004;49:4583–8.
Yekeler H, Guven A, Ozkan R. Hydrogen bonding and dimeric self-association of 2-pyrrolidinone: An ab initio study. J Comput Aided Mol Des. 1999;135:89–596.
Jouyban A, Fakhree MAA, Shayanfar A. Review of pharmaceutical applications of N-Methyl-2-Pyrrolidone. J Pharm Pharmaceut Sci. 2010;13(4):524–35.
Rathnam MV, Sayed RT, Bhanushali KR, Kumar MSS. Density and viscosity of binary mixtures of n-butyl acetate with ketones at (298.15, 303.15, 308.15, and 313.15) K. J Chem Eng Data. 2012;57:1721–7.
Tsierkezos NG, Molinou IE, Polizos GA. Relative permittivities, speeds of sound, viscosities, and densities of cyclohexanone +cis-decalin and cyclohexanone +trans-decalin mixtures at 283.15, 293.15, and 303.15 K. J Chem Eng Data. 2002;47:1492–5.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroborate and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Sharma D, Bhagour S, Sharma VK. Thermodynamic and topological studies of 1-ethyl-3-methylimidazolium tetrafluoroborate + pyrrolidin-2-one and 1-methyl-pyrrolidin-2-one mixtures. J Chem Eng Data. 2012;57:3488–97.
Sharma VK, Kataria J, Solanki S. Molecular interactions in binary mixtures of lactams with cyclic alkanones. J Solution Chem. 2014;43:486–524.
Scholz E. Karl Fischer titration. Berlin: Springer; 1984.
Garcia B, Herrera C, Leal JS. Shear viscosities of binary liquid mixtures: 2-pyrrolidinone with 1-alkanols. J Chem Eng Data. 1991;36:269–74.
Letcher TM, Lachwa J, Domanska U. The excess molar volumes and enthalpies of (N-methyl-2-pyrrolidinone + an alcohol) at T = 298.15 K and The application of the ERAS theory. J Chem Thermodyn. 2001;33:1169–79.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley Interscience; 1986.
Curras MR, Gomes MFC, Husson P, Padua AAH, Garcia J. Calorimetric and volumetric study on binary mixtures 2,2,2-trifluoroethanol + (1-Butyl-3-methylimidazolium tetrafluoroborate or 1-ethyl-3-methylimidazolium tetrafluoroborate). J Chem Eng Data. 2010;55:5504–12.
Navia P, Troncoso J, Romani L. Excess magnitudes for ionic liquid binary mixtures with a common ion. J Chem Eng Data. 2007;52:1369–74.
Stoppa A, Zech O, Kunz W, Buchner R. The conductivity of imidazolium-based ionic liquids from (−35 to 195) °C. A. Variation of Cation’s Alkyl Chain. J Chem Eng Data. 2010;55:1768–73.
Pal A, Bhardwaj RK. Excess molar volumes and viscosities for binary mixtures of 2-propoxyethanol and of 2-isopropoxyethanol with 2-pyrrolidinone, N-methyl-2-pyrrolidinone, N, N-Dimethylformamide, and N,N-Dimethylacetamide at 298.15 K. J Chem Eng Data. 2002;47:1128–34.
Papamatthaiakis D, Aroni F, Havredaki V. Isentropic compressibilities of (amide + water) mixtures: a comparative study. J Chem Thermodyn. 2008;40:107–18.
Garcia-Abuin A, Gomez-Diaz D, Rubia MDL, Navaza JM. Density, speed of sound, viscosity, refractive index, and excess volume of N-methyl-2-pyrrolidone + ethanol (or water or ethanolamine) from T = (293.15 to 323.15) K. J Chem Eng Data. 2011;56:646–51.
Kumari PG, Radhamma M, Sekhar GC, Rao MV. Excess volumes and speeds of sound of N-methyl-2-pyrrolidone with chloroethanes and chloroethenes at 303.15 K. J Chem Eng Data. 2002;47:425–7.
Changsheng Y, Peisheng MA, Qing Z. Excess molar volumes and viscosities of binary mixtures of p-xylene with cyclohexane, n-heptane, n-octane, sulfolane, N-methyl-2-pyrrolidinone and acetic acid at 303.15 K and 323.25 K and atmospheric pressure. Chinese J Chem Eng. 2004;12:700–6.
Ciocirlan O, Teodorescu M, Dragoesce D, Iulian O, Barhala A. Densities and excess molar volumes of the binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15, and 318.15) K. J Chem Eng Data. 2010;55:3891–5.
Dragoescu D, Teodorescu M, Barhala A. Isothermal (vapour plus liquid) equilibria and excess Gibbs free energies in some binary (cyclopentanone plus chloroalkane) mixtures at temperatures from 298.15 to 318.15 K. J Chem Thermodyn. 2007;39:1452–7.
Palaiologou MM, Arianas GK, Tsierkezos NG. Thermodynamic investigation of dimethyl sulfoxide binary mixtures at 293.15 and 313.15 K. J Solution Chem. 2006;35:1551–65.
Lange NA. Handbook of Chemistry. 11th ed. New York: Mc Graw-Hill; 1973.
Nayak JN, Aralaguppi MI, Aminabhavi TM. Density, viscosity, refractive index, and speed of sound in the binary mixtures of 1,4-dioxane + ethyl acetoacetate, + diethyl oxalate, + diethyl phthalate, or + dioctyl phthalate at 298.15, 303.15, and 308.15 K. J Chem Eng Data. 2003;48:1489–94.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes for binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Singh S, Rattan VK, Kapoor S, Kumar R, Rampal A. Thermophysical properties of binary mixtures of cyclohexane + nitrobenzene, cyclohexane + nitrobenzene, and cyclohexane + cyclohexanone at (298.15, 303.15, and 308.15) K. J Chem Eng Data. 2005;50:288–92.
Rafiee HR, Ranjbar S, Poursalman F. Densities and viscosities of binary and ternary mixtures of cyclohexanone, 1,4-dioxane and isooctane from T = (288.15 to 313.15) K. J Chem Thermodyn. 2012;54:266–71.
George J, Sastry NV. Densities, viscosities, speeds of sound, and relative permittivities for water + cyclic amides (2-pyrrolidinone, 1-methyl-2-pyrrolidinone and 1-vinyl-2-pyrrolidinone at different temperatures. J Chem Eng Data. 2004;49:235–42.
Bermudez-Salguero C, Gracia-Fadrique J, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Tsierkezos NG, Molinou IE, Filippou AC. Thermodynamic properties of binary mixtures of cyclohexanone with n-alkanols (C1-C5) at 293.15 K. J Solution Chem. 2005;34:1371–86.
Sanmamed YA, Navia P, Salgado DG, Troncoso J, Romaní L. Pressure and temperature dependence of isobaric heat capacity for [Emim][BF4], [Bmim][BF4], [Hmim][BF4] and [Omim][BF4]. J Chem Eng Data. 2010;55:600–4.
Nishikawa K, Ohomura K, Tamura K, Murakami S. Excess thermodynamic properties of mixtures of cyclohexanone and benzene at 298.15 and 308.15 K and the effect of excess expansion factor. Thermochim Acta. 1995;267:323–32.
Neeti Yadav JS, Jangra SK, Dimple Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sabbah R, Xu-wu A, Chickos JS, Leitão MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:137.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Huggins ML. The thermodynamic properties of liquids included solutions: part 1. Intermolecular energies in mono atomic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids included solutions: part 2. Polymer solutions considered as diatomic system. Polymer. 1971;12:389–99.
Singh PP, Bhatia M. Energetic of molecular interactions in binary mixtures of non-electrolytes containing a salt. J Chem Soc Faraday Trans. 1989;I(85):3807–12.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–90.
Yadav JS, Sharma D, Sharma VK. Topological investigations of thermodynamic properties of binary mixtures containing 2-pyrrolidinone. Thermochim Acta. 2009;489:45–52.
Sharma VK, Siwach RK. Dimple. Excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities of tetrahydropyran with aromatic hydrocarbons tetrahydropyran with aromatic hydrocarbons. J Chem Thermodyn. 2011;43:39–46.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Kier LB, Yalkowasky SH, Sinkula AA, Valvani SC. Physico-chemical properties of drugs. Mercel Dekker. New York chapter 9. 1980, (a) p. 282, (b) p. 295.
Van HT, Patterson D. Volumes of mixing and the P * effect: part I. Hexane isomers with normal and branched hexadecane. J Solut Chem. 1982;11:793–805.
Flory PJ. The statistical thermodynamic of liquid mixtures. J Am Chem Soc. 1965;87:1833–8.
Flory PJ. The thermodynamic properties of mixture of small non-polar molecules. J Am Chem Soc. 1965;87:1838–46.
Acknowledgements
Jyoti Kataria is grateful to UGC, New Delhi, India, for the award of SRF. The authors are also grateful to the Head of Chemistry Department and authorities of M. D. University, Rohtak, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V.K., Kataria, J. & Solanki, S. Excess molar enthalpies for ternary mixtures containing [emim][BF4], cyclic amides and cyclic ketones. J Therm Anal Calorim 123, 1571–1582 (2016). https://doi.org/10.1007/s10973-015-5033-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5033-y