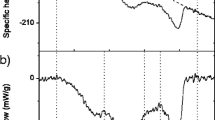
Abstract
The application of thermal analysis to meat products, like all materials, is governed by energy transfer, from which invaluable information on thermodynamics of processes is obtained. Various meat systems have been analyzed with different success ratios. Proteins in meat formed the key area of interest due to their effect on product quality, shelf life, functionality and also their ability to interact with other components. Initially success rates were low owing to lack of sensitivity, results with complex curves, complications in interpretation and a large number of factors affecting the biological system. Later with developments in instrumentation and computer models for better resolution and reproducibility, wider horizons could be covered with easy solutions to complex results. Though thermal analysis has been used exhaustively in meat, research in this area has become stagnant and new areas are being left untouched. This review emphasizes the importance of thermal analysis in the meat industry and also the need to use more advanced equipment for better understanding of the biological changes occurring in muscle during processing and storage.


Similar content being viewed by others
References
Alvarado JDD. Specific heat of dehydrated pulps of fruits. J Food Process Eng. 1991;14(3):189–96.
Rizvi SSH, Singh RK, Hotchkiss JH, Heldman DR, Leung H. Research needs in food engineering, processing, and packaging. Food Technol. 1993;47:26s–35s.
Ozawa T. Thermal analysis—Review and prospect. Thermochim Acta. 2000;355:35–42.
Cheng SZ, Li CY, Calhoun BH, Zhu L, Zhou WW. Thermal analysis: the next two decades. Thermochim Acta. 2000;355(1):59–68.
Mackenzie RC, Mitchell BD. Differential thermal analysis. A review. Analyst. 1962;87(1035):420–34.
Ma CY, Harwalkar VR. Thermal analysis of food proteins. Adv Food Nutr Res. 1991;35:317–66.
Connolly M, Tobias B. Analysis of thermoset curve by dynamic mechanical, dielectric, and calorimetric methods. Am Lab. 1992; 24(1): 38, 40–42.
DiVito MP, Fielder KJ, Curran GH, Feder MS. Recent advances in routine thermal analysis instrumentation. Am Lab. 1992; 24(1): 30, 32, 34–36.
ASHRAE. ASHRAE handbook-fundamentals. New York: The American Society of Heating, Refrigerating and Air Conditioning Engineers; 1985.
Polley SL, Snyder OP, Kotnour P. A compilation of thermal properties of foods. Food Technol. 1980; 34 (11): 76–80, 82–84, 86–88, 90–92, 94.
Sweat VE. Thermal properties of foods. In: Rao MA, Rizvi SSH, editors. Engineering properties of foods. 2nd ed. New York: Marcel Dekker Inc; 1995. p. 99–167 Chapter 4.
Plotnikov VV, Brandts JF, Brandts JM. U.S. Patent No. 6,869,214. 2005; Washington, DC: U.S. Patent and Trademark Office.
Wang L, Zhao Y, Ng E, Lin Q. A MEMS differential calorimeter for biomolecular characterization. In: Micro electro mechanical systems, 2005. MEMS 2005. 18th IEEE international conference. 2005; 814–817. IEEE.
Danley RL. Infrared heated differential scanning calorimeter. U.S. Patent No. 8,087,821. 2012; Washington, DC: U.S. Patent and Trademark Office.
Vlassak JJ, McCluskey PJ. Parallel nano-differential scanning calorimetry. U.S. Patent 2007 286 769 A1. 2007.
Holland BJ, Atkinson JR, Hay JN. Design and development in self-reference differential scanning calorimetry. J Therm Anal Calorim. 2002;69:371–85.
Mathota VBF, Vanden Poel G, Pijpers TFJ. Benefits and potentials of high performance differential scanning calorimetry (HPer DSC). In: Brown M, Gallagher P, editors. Handbook of thermal analysis and calorimetry, vol. 5. The Netherlands: Elsevier; 2007. p. 269–97.
Reading M. Method and apparatus for gas flow modulated differential scanning calorimetry. US Patent No. 5,624,187. 29. 1997.
Cannon JE, Morgan JB, Heavner J, McKeith FK, Smith GC, Meeker DL. Pork quality audit: a review of the factors influencing pork quality. J Muscle Foods. 1995;6:369–402.
Barbut S, Findlay C. Influence of sodium, potassium and magnesium chloride on thermal properties of beef muscle. J Food Sci. 1991;56:180–2.
Samejima K, Ishioroshi M, Yasui T. Scanning calorimetric studies on the thermal denaturation of myosin and its sub fragments. Agric Biol Chem. 1983;47:2373–9.
King NL, Harris PV. Heat-induced tenderization of meat by endogenous carboxyl proteases. Meat Sci. 1982;6:137–48.
Martens H, Stabursvik E, Martens M. Texture and color changes in meat during cooking related to thermal denaturation of muscle proteins. J Textural Stud. 1982;13:291–309.
Seuss IE, Pospiech E, Honikel KO. Causes of cooking loss on heating of meat. In: Proceedings of the 32nd European meeting of meat research workers. 1986; 4: 143–146.
Saadoun A, Cabrera MC. A review of the nutritional content and technological parameters of indigenous sources of meat in South America. Meat Sci. 2008;80:570–81.
Demirel G, Ozpinar H, Nazli B, Keser O. Fatty acids of lamb meat from two breeds fed different forage: concentrate ratio. Meat Sci. 2006;72(2):229–35.
Raemy A, Lambelet P. Thermal behavior of foods. Thermochim Acta. 1991;193:417–39.
Wright DJ, Leach IB, Wilding P. Differential scanning calorimetric studies of muscle and its constituent proteins. J Sci Food Agric. 1977;28(6):557–64.
Park JW, Lanier TC. Calorimetric changes during development of rigor mortis. J Food Sci. 1988;53(1312–1314):1372.
Hamm R, Deatherage FE. Changes in hydration solubility and charges of muscle proteins during heating of meat. Food Res. 1960;25:587.
Bouton PE, Harris PV, Shorthose WR. Dimensional changes in meat during cooking. J Texture Stud. 1976;7:179–92.
Bowers JA, Craig JA, Kropf DH, Tucker TJ. Flavor, color and other characteristics of beef longissimus muscle heated to seven internal temperatures between °C and 85 °C. J Food Sci. 1987;52:533–6.
Stabursvik E, Fretheim K, Froystein T. Myosin denaturation in pale, and exudative (PSE) porcine muscle tissue as studied by differential scanning calorimetry. J Sci Food Agric. 1983;35(2):240–4.
Thorarinsdottir KA, Arason S, Geirsdottir M, Bogason S, Kristbergsson K. Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chem. 2002;77:377–85.
Findlay CJ, Parkin KL, Stanley DW. Differential scanning calorimetry can determine kinetics of thermal denaturation of beef muscle proteins. J Food Biochem. 1986;10(1):1–15.
Stabursvik E, Martens H. Thermal denaturation of proteins in post rigor muscle tissue as studied by differential scanning calorimetry. J Sci Food Agric. 1980;31(10):1034–42.
Wright D, Winding P. Differential scanning calorimetric study of muscle and its proteins: myosin and its subfragments. J Sci Food Agric. 1984;35(3):357–71.
Ledward DA, Chizzolini R, Lawrie RA. The effect of extraction, animal age and postmortem storage on tendon collagen. A differential scanning calorimetric study. J Food Technol. 1975;10:349–54.
Hellauer H, Winkler R. Denaturation of collagen fibers in NaI, NaCl and water of different pH values as studied by differential scanning calorimetric measurements. Connect Tissue Res. 1975;3:227–35.
Ledward DA, Lawrie RA. A note on the dependence of meat texture on the temperature of measurement. J Food Sci Agric. 1975;26:691–5.
Martens H, Vold E. DSC studies of muscle tissue protein denaturation. In: congress documentation; proceedings of the European meeting of meat research workers. 1976; Vol. 1976.
Kijowski JM, Mast MG. Thermal properties of proteins in chicken broiler tissues. J Food Sci. 1988;53:363–6.
Xiong YL, Brekke CJ, Leung HK. Thermal denaturation of muscle proteins from different species and muscle types as studied by differential scanning calorimetry. Can Inst Food Sci Technol J. 1987;20:357–62.
Murphy RY, Marks BP, Marcy JA. Apparent specific heat of chicken breast patties and their constituent proteins by differential scanning calorimetry. J Food Sci. 1998;63(1):88–91.
Demopoulos CA, Antonopoulou S, Andrikopoulos NK, Kapoulas VM. Isolation and complete separation of lipids from natural sources. J Liq Chromatogr Relat Technol. 1996;19(4):521–35.
Yılmaz MT, Karakaya M. Thermal analysis of lipids isolated from various tissues of sheep fats. J Therm Anal Calorim. 2010;101(1):403–9.
Skala D, Milovanović L, Ranić M, Katsikas L, Bastić M. The thermal analysis of lipids isolated from various tissues of deers and does. J Therm Anal. 1997;49(2):869–77.
van Aken GA, Ten Grotenhuis E, Van Langevelde AJ, Schenk H. Composition and crystallization of milk fat fractions. J Am Oil Chem Soc. 1999;76(11):1323–31.
Milovanović LM, Popović I, Skalać D, Saićicć S. Thermogravimetric analysis of the total lipids extracted from the fatty tissue of fallow deer (Cervus Dama dama L). J Serbian Chem Soc. 2006;71(12):1281–88.
Teixeira GA, Maia AS, Rosenhaim R, Santos IM, Souza AL, Souza AG, Queiroz N. Thermo-oxidative decomposition of biodiesel samples obtained from mixtures of beef tallow, soybean oil, and babassu oil. J Therm Anal Calorim. 2011;106(2):569–74.
Ramalho EFSM, Santos IMG, Maia AS, Souza AL, Souza AG. Thermal characterization of the poultry fat biodiesel. J Therm Anal Calorim. 2011;106(3):825–9.
Fernandez-Martin F, Jimenez Colmenero F. Pressure/temperature processing of low and high fat frankfurters: denaturation effects on the proteins. Proceedings 44th international congress of meat science and technology II. 1998; 546–547.
Warriss PD. Meat science: an introductory text. Wallingford: CAB-International; 2000.
Barbut S, Zhang L, Marcone M. Effects of pale, normal and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poult Sci. 2005;84:797–802.
Swatland HJ. How pH causes paleness or darkness in chicken breast meat. Meat Sci. 2008;80:396–400.
Viljoena HF, de Kocka HL, Webb EC. Consumer acceptability of dark, firm and dry (DFD) and normal pH beef steaks. Meat Sci. 2002;61:181–5.
Bartos L, Franc C, Rehák D, Stípková L. A practical method to prevent dark-cutting (DFD) in beef. Meat Sci. 1993;34:275–82.
Kreikemeier KK, Unruh JA, Eck TP. Factors affecting the occurrence of dark-cutting beef and selected carcass traits in finished beef cattle. J Anim Sci. 1998;76:388–95.
Mounier L, Dubroeucq H, Andanson S, Veissier I. Variations in meat pH of beef bulls in relation to conditions of transfer to slaughter and previous history of the animals. J Anim Sci. 2006;84:1567–76.
Stabursvik E, Fretheim K, Froystein T. Myosin denaturation in pale, soft, and exudative (PSE) porcine muscle tissue as studied by differential scanning calorimetry. J Sci Food Agric. 1984;35:240–4.
Borzuta K, Borys A, Grześkowiak E, Wajda S, Strzelec ki J, Lisiak DX. Variability of slaughter value and meat quality of fatteners slaughtered in summer of 2002. Roczn. IPMiT. 1984;40:5–11.
Voutila L, Mullen AM, Allen P, Troy D, Puolanne E. Thermal stability of connective tissue and meat quality of loose structured porcine semimembranosus muscles. Maataloustieteen Päivät. 2006.
Xiong YL, Brekke CJ. Thermal transitions of salt-soluble proteins from pre- and postrigor chicken muscles. J Food Sci. 1990;55(1540–1543):1570.
Whiting RC, Richards JF. Can Inst Food Sci Technol J. 1975;8:168.
Drăghici O. Determination by DSC analysis of the influence of pH on meat proteins. J Agroaliment Process Technol. 2011;17(4):466–8.
Mirsky AE. The change in state of the proteins of muscle in rigor. J Gen Physiol. 1936;19:559–71.
Feiner G. Meat products handbook: practical science and technology. Woodhead Publishing series in food science, technology and nutrition; 2006.
Winger RJ, Pope CG. Osmotic properties of post-rigor beef muscle. Meat Sci. 1980–1981; 5: 355–369.
Geesink GH, Koohmaraie M. Postmortem proteolysis and calpain/calpastatin activity in callipyge and normal lamb biceps femoris during extended postmortem storage. J Anim Sci. 1999;77:1490–501.
Huff-Lonergan E, Lonergan SM. Postmortem mechanisms of meat tenderization: the roles of the structural proteins and the calpain system. In: Xiong YL, Ho CT, Shahidi F, editors. Quality attributes of muscle food. New York: Kluwer Academic/Plenum Publishers; 1999. p. 229–51.
Martinaud A, Mercier Y, Marinova P, Tassy C, Gatellier P, Renerre M. Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems. J Agric Food Chem. 1997;45:2481–7.
Offer G, Knight P. The structural basis of water-holding capacity in meat. Part 1: general principles and water uptake in meat processing. In: Lawrie R, editor. Developments in meat science, vol. 4. New York: Elsevier Applied Science; 1988. p. 61–171.
Offer G, Knight P. The structural basis of water-holding capacity in meat. Part 2: drip losses. In: Lawrie R, editor. Developments in meat science, vol. 4. London: Elsevier Science Publications; 1988. p. 173–243.
Savage AWJ, Warriss PD, Jolley PD. The amount and composition of the proteins in drip from stored pig meat. Meat Sci. 1990;27:289–303.
Offer G, Trinick J. On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci. 1983;8:245–381.
Quinn JR, Raymond DP, Harwalkar VR. Differential scanning calorimetry of meat proteins as affected by processing treatment. J Food Sci. 1980;45:1146–9.
Graiver N, Pinotti A, Califano A, Zaritzky N. Diffusion of sodium chloride in pork tissue. J Food Eng. 2006;77(4):910–8.
Findlay CJ, Stanley DW. Differential scanning calorimetry of beef muscle: influence of postmortem conditioning. J Food Sci. 1984;49(6):1513–6.
Xiong YL. Structure-function relationships of muscle proteins. In: Damodaran S, Paraf A, editors. Food proteins and their applications. USA: CRC Press; 1997.
Oda T, Makino K, Yamashita I, Namba K, Maéda Y. Distinct structural changes detected by X-ray fiber diffraction in stabilization of F-actin by lowering pH and increasing ionic strength. Biophys J. 2001;80(2):841–51.
Aktas N, Kaya M. Detection of beef body fat and margarine in butterfat by differential scanning calorimetry. J Therm Anal Calorim. 2001;66:795–801.
Froning GW. Effect of various additives on the binding properties of chicken meat. Poult Sci. 1966;45:185.
Kijowski JM, Mast MGE. Effect of sodium chloride and phosphates on the thermal properties of chicken meat proteins. J Food Sci. 1988b; 53(2): 367–370, 387.
Tomaszewska-Gras J, Konieczny PA. DSC study on the effect of marination on the stability of skin collagen from chicken wings. Acta Sci Pol Technol Aliment. 2010;9(4):413–23.
Bendall JR, Restall DJ. The cooking of single myofibrils, small myofibril bundles and muscle strip from beef M. psoas and M. sternomandibularis muscles at varying heating rates and temperatures. Meat Sci. 1983;8:93–117.
Stanley DW, Swatland HJ. The microstructure of muscle tissue—A basis for meat texture measurement. J Texture Stud. 1976;7(1):65–75.
Borchardt HJ, Daniels F. The application of differential analysis to the study of reaction kinetics. J Am Chem Soc. 1956;79:41–6.
Findlay CJ, Stanley DW, Gullett EA. Thermomechanical properties of beef muscle. Meat Sci. 1986;22:57–70.
Bailey AJ. Recent advances in the chemistry of meat. London: The Royal Society of Chemistry; 1984.
Fukazawa T, Hashimoto Y, Yasui T. Effect of storage conditions on some physicochemical properties in experimental sausage prepared from fibrils. J Food Sci. 1961;26:331–6.
Fukazawa T, Hashimoto Y, Yasui T. Effect of some proteins on the binding quality of an experimental sausage. J Food Sci. 1961;26:541–9.
Fukazawa T, Hashimoto Y, Yasui T. The relationship between the components of myofibrillar protein and the effect of various phosphates that influence the binding quality of sausage. J Food Sci. 1961;26:550–5.
Samejima K, Hashimoto Y, Yasui T, Fukazawa T. Heat gelling properties of myosin, actin, actomyosin and myosin-subunits in a saline model system. J Food Sci. 1969;34:242–5.
Yasui T, Fukazawa T, Takahashi K, Sakanishi M, Hashimoto Y. Specific interaction of inorganic polyphosphates with myosin B. J Agric Food Chem. 1964;12:399–404.
Hermansson AM, Langton M. Filamentous structures of bovine myosin in diluted suspensions and gels. J Sci Food Agric. 1988;42:355–69.
Hamm R, Grabowska J. Proteinlö slichkeit und Wasserbindung unter den in Brühwurstbräten gegebenen Bedingungen. Die Fleischwirtschaft. 1978;58:1345–7.
Yasui T, Ishioroshi M, Samejima K. Heat-induced gelation of myosin in the presence of actin. J Food Biochem. 1980;4(2):61–78.
Doerscher DR, Briggs JL, Lonergan SM. Effects of pork collagen on thermal and viscoelastic properties of purified myofibrillar protein gels. Meat Sci. 2003;66:181–8.
Fretheim K, Egelandsdal B, Harbitz O, Samejima K. Slow lowering of pH induces gel formation of myosin. Food Chem. 1985;18:169–78.
Fernández-Martın F, Cofrades S, Carballo J, Jiménez-Colmenero F. Salt and phosphate effects on the gelling process of pressure/heat treated pork batters. Meat Sci. 2002;61(1):15–23.
Townsend WE, Ackerman SA, Witnauer LP, Palm WE, Swift CE. Effect of types and level of fat and rates and temperature of comminution on the processing and characteristics of frankfurters. J Food Sci. 1971;36:261–5.
Whiting RC. Ingredients and processing factors that control muscle protein functionality. Food Technol. 1988;42(4):104–14.
Park J, Rhee KS, Keeton JT, Rhee KC. Properties of low-fat frankfurters containing monounsaturated and omega-3 polyunsaturated oils. J Food Sci. 1989;54(3):500–4.
Bater B, Maurer AJ. Effects of fat source and final comminution temperature on fat particle dispersion, emulsion stability, and textural characteristics of turkey frankfurters. Poult Sci. 1991;70:1424–9.
Barreto G, Carballo J, Fernandez-Martín F, Colmenero FJ. Thermal gelation of meat batters as a function of type and level of fat and protein content. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1996;202(3):211–4.
Doerscher DR, Briggs JL, Lonergan SM. Effects of pork collagen on thermal and viscoelastic properties of purified porcine myofibrillar protein gels. Meat Sci. 2004;66(1):181–8.
Westphalen AD, Briggs JL, Lonergan SM. Influence of pH on rheological properties of porcine myofibrillar protein during heat induced gelation. Meat Sci. 2005;70(2):293–9.
Ahmed J, Ramaswamy HS. Dynamic rheology and thermal transitions in meat-based strained baby foods. J Food Eng. 2007;78(4):1274–84.
Samejima K, Ishioroshi M, Yasui T. Relative roles of the head and tail portions of the molecule in heat-induced gelation of myosin. J Food Sci. 1981;46(5):1412–8.
Sano T, Noguchi SF, Tsuchiya T, Matsumoto JJ. Dynamic viscoelastic behavior of natural actomyosin and myosin during thermal gelation. J Food Sci. 1988;53(3):924–8.
Ensor SA, Sofos JN, Schmidt GR. Differential scanning calorimetric studies of meat protein alginate mixtures. J Food Sci. 1991;56(1):175–9.
Shand PJ, Sofos JN, Schmidt GR. Differential scanning calorimetry of beef/kappa-carrageenan mixtures. J Food Sci. 1994;59(4):711–5.
DeFreitas Z, Sebranek JG, Olson DG, Carr JM. Carrageenan effects on thermal stability of meat proteins. J Food Sci. 1997;62(3):544–7.
Donatus ENA, Xiong YL. Effects of carrageenan on thermal stability of proteins from chicken thigh and breast muscles. Food Res Int. 2001;34:247–53.
Irie M. Evaluation of porcine fat with fiber-optic spectroscopy. J Anim Sci. 1999;77:2680–3.
Irie M, Swatland HJ. Assessment of porcine fat quality by fiber-optic spectrophotometry. Asian-Australas J Anim Sci. 1992;75:753–6.
Keller G, Lavigne F, Loisel C, Ollivon M, Bourgaux C. Investigation of the complex thermal behavior of fats. J Therm Anal Calorim. 1996;47(5):1545–65.
Loisel C, Keller G, Lecq G, Bourgaux C, Ollivon M. Phase transitions and polymorphism of cocoa butter. J Am Oil Chem Soc. 1998;75(4):425–39.
Wong NP. Fundamentals of dairy chemistry. 3rd ed. Gaithersburg: Aspen Publishers; 1999. p. 779.
Saeed S, Fawthrop SA, Howell NK. Electron spin resonance (ESR) study on free radical transfer in fish lipid–protein interaction. J Sci Food Agric. 1999;79(13):1809–16.
Milovanović LM, Popović I, Ranić MR, Saičić S, Skala D, Antonović D. Total lipids of the intramuscular tissue of fallow deer. J Therm Anal Calorim. 2007;89(3):929–34.
Saeed S, Howell NK. Rheological and differential scanning calorimetry studies on structural and textural changes in frozen Atlantic mackerel (Scomber scombrus). J Sci Food Agric. 2004;84(10):1216–22.
Marikkar JMN, Lai OM, Ghazali HM, Man YC. Detection of lard and randomized lard as adulterants in refined-bleached-deodorized palm oil by differential scanning calorimetry. J Am Oil Chem Soc. 2001;78(11):1113–9.
de Man JM. Functionality requirements of fats and oils for food application, presented at MOSTA Tech-In. Recent advances in the sciences of oils and fats. 1999.
Yılmaz MT, Karakaya M, Aktaş N. Composition and thermal properties of cattle fats. Eur J Lipid Sci Technol. 2010;112(3):410–6.
Marikkar JMN, Lai OM, Ghazali HM. Chen Man YB. Compositional and thermal analysis of RBD palm oil adulterated with lipase-catalyzed interesterified lard. Food Chem. 2002;76:249–58.
Marikkar JMN, Ghazali HM, Chen Man YB, Lai OM. Differential scanning calorimetric analysis for determination of some animal fats as adulterants in palm olein. J Food Lipids. 2003;10:63–79.
Coni E, Di Pasquale M, Coppolelli P, Bocca A. Detection of animal fats in butter by differential scanning calorimetry: a pilot study. J Am Oil Chemists’ Soc. 1994;71(8):807–10.
Marikkar JMN, Ghazali HM, Long K, Lai OM. Lard uptake and its detection in selected food products deep-fried in lard. Food Res Int. 2003;36:1047–60.
Ikeuchi Y, Ito T, Fukazawa T. A kinetic analysis of thermal denaturation of F-actin. Int J Biochem. 1981;13:1065–9.
Chiu J, Fair PG. Determination of thermal conductivity by differential scanning calorimetry. Thermochim Acta. 1979;34(2):267–73.
Wagner JR, Anon MC. Denaturation kinetics of myofibrillar proteins in bovine muscle. J Food Sci. 1985;50(6):1547–50.
Kajitani S, Fukuoka M, Sakai N. Kinetics of thermal denaturation of protein in cured pork meat. Jpn J Food Eng. 2011;12(1):19–26.
Mortensen M, Andersen HJ, Engelsen SB, Bertram HC. Effect of freezing temperature, thawing and cooking rate on water distribution in two pork qualities. Meat Sci. 2006;72(1):34–42.
Ngapo TM, Babare IH, Reynolds J, Mawson RF. Freezing and thawing rate effects on drip loss from samples of pork. Meat Sci. 1999;53(3):149–58.
Do GS, Sagara Y, Tabata M, Kudoh KI, Higuchi T. Three-dimensional measurement of ice crystals in frozen beef with a micro-slicer image processing system. Int J Refrig. 2004;27(2):184–90.
Bevilacqua AE, Zaritzky NE. Ice morphology in frozen beef. Food Technol. 1980;15:589–97.
Bertram HC, Andersen RH, Andersen HJ. Development in myofibrillar water distribution of two pork qualities during 10-month freezer storage. Meat Sci. 2007;75(1):128–33.
Sawyer JT, Baublits RT, Apple JK, Meullenet JF, Johnson ZB, Alpers TK. Lateral and longitudinal characterization of color stability, instrumental tenderness, and sensory characteristics in the beef semimembranosus. Meat Sci. 2007;75(4):575–84.
Meléndez-Pérez R, Arjona-Román JL, Velázquez-Castillo RR, Méndez-Albores A, Vázquez-Durán A. On the thermal properties of frozen, refrozen and freeze drying porcine Logissimusdorsi. J Anim Vet Adv. 2011;10(22):2956–60.
Mietsch F, Halász A, Farkas J. Untersuchung über Ä nderungen von Fleischproteinen während der gefrierlagung. Die Nahrung. 1994;38:47–52.
Aktaş N, Tülek Y, Gökalp HY. Determination of differences in free and bound water contents of beef muscle by DSC under various freezing conditions. J Therm Anal. 1997;50(4):617–24.
Aktas N, Tülek Y, Gökalp HY. Determination of freezable water content of beef semimembranous muscle DSC study. J Therm Anal Calorim. 1997;48(2):259–66.
Fernández-Martín F, Otero L, Solas MT, Sanz PD. Protein denaturation and structural damage during high-pressure-shift freezing of porcine and bovine muscle. J Food Sci. 2000;65(6):1002–8.
Messens W, Van Camp J, Huyghebaert A. Use of high pressure to modify the functionality of food proteins. Trends Food Sci Technol. 1997;8:107–12.
Molina E, Papadopoulou A, Ledward DA. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocolloids. 2001;15:263–9.
Apichartsrangkoon A, Ledward DA, Bell AE, Brennan JG. Physicochemical properties of high pressure treated wheat gluten. Food Chem. 1998;63(2):215–20.
Fernandez-Martin F. Bird muscles under hydrostatic high-pressure/temperature combinations. J Therm Anal Calorim. 2007;87(1):285–90.
Macfarlane JJ, Mckenzie IJ, Turner RH. Pressure treatment of meat: effects on thermal transitions and shear values. Meat Sci. 1980;5:307–17.
Fernández-Martín F, Fernández P, Carballo J, Colmenero FJ. Pressure/heat combinations on pork meat batters: protein thermal behavior and product rheological properties. J Agric Food Chem. 1997;45:4440–5.
Fernandez-Martin F, Sanz PD, Otero L. Pressure assisted freezing of pork muscle meat Vs ordinary freezing: protein denaturation. In: Ludwig H, editor. Advances in high-pressure and biotechnology. Berlin: Springer; 1999. p. 468–72.
Fernández-Martín F, Fernández P, Carballo J, Jiménez-Colmenero F. DSC study on the influence of meat source, salt and fat levels, and processing parameters on batters pressurization. Eur Food Res Technol. 2000;211(6):387–92.
Supavititpatana T, Apichartsrangkoon A. Combination effects of ultra-high pressure and temperature on the physical and thermal properties of ostrich meat sausage (yor). Meat Sci. 2007;76(3):555–60.
Parés D, Saguer E, Saurina J, Suñol JJ, Toldrà M, Carretero C. DSC study of the effects of high pressure and spray-drying treatment on porcine plasma. J Therm Anal Calorim. 1998;52(3):837–44.
Hamm R. In: Höyem T, Kvåle O, (eds) (1977) Physical, chemical and biological changes in food caused by thermal processing. Applied Science Publishers: London. pp. 101–134.
Tornberg E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508.
Offer G. Progress in the biochemistry, physiology and structure of meat. In: Proceedings from the 30th European meeting of meat research workers. Bristol; 1984. pp. 87–93.
Bejerholm C, Aaslyng MD. The influence of cooking technique and core temperature on results of sensory analysis of pork depending on the raw meat quality. Food Qual Prefer. 2003;15:19–30.
Joseph JK, Awosanya B, Adeniran AT, Otagba UM. The effect of end-point internal cooking temperatures on the meat quality attributes if selected Nigerian poultry meats. Food Qual Prefer. 1997;8:57–61.
Aaslyng MD, Bejerholm C, Ertbjerg P, Bertram HC, Andersen HJ. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual Prefer. 2003;14:277–88.
Wood JD, Nute GR, Fursey GA, Cuthbertson A. The effect of cooking conditions on the eating quality of pork. Meat Sci. 1995;40:127–35.
Bertram HC, Wu Z, van den Berg F, Andersen HJ. NMR relaxometry and differential scanning calorimetry during meat cooking. Meat Sci. 2006;74(4):684–9.
Greaser ML. Postmortem changes in the cytoskeleton proteins in muscle. Proc. XIII Europ. Symposium ‘‘the quality of poultry meat’’. Poznań. 1997; 1: 281–291.
Fritz JD, Dietrich LJ, Greaser ML. Cooking effects on titin in fresh and processed beef products. J Muscle Foods. 1992;3:133–40.
King NL. Breakdown of connectin during cooking of meat. Meat Sci. 1984;11:27–43.
Pospiech E, Greaser ML, Mikolajczak B, Chiang W, Krzywdzińska M. Thermal properties of titin from porcine and bovine muscles. Meat Sci. 2002;62(2):187–92.
Oliveira JC, Pereira PM, Frias JM, Cruz IB, MacInnes WM. Application of the concepts of biomaterials science to the quality optimization of frozen foods. In: Oliveira FAR, Oliveira JC, editors. Processing foods quality optimization and process assessment. USA: CRC Press LLC; 1999. p. 107–40.
Roos Y. Water activity and glass transition temperature: How do they complement and how do they differ. In: Barbosa-Canovas GV, Welti-Chanes J, editors. Food preservation by moisture control fundamentals and applications. USA: Technomic Publishing; 1995. p. 133–54.
Grunina NA, Belopolskaya TV, Tsereteli TI. The glass transition process in humid biopolymers.DSC study. J Phys: Conf Ser. 2006;40(1):105.
Goff HD. Measuring and interpreting the glass transition in frozen foods and model systems. Food Res Int. 1994;27:187–9.
Simatos D, Blond G, Perez J. Basic physical aspects of glass transition. In: Barbosa-Canovas GV, Welti-Chanes J, editors. Food preservation by moisture control fundamentals and applications. USA: Technomic Publishing; 1995. p. 3–31.
Delgado AE, Sun DW. Desorption isotherms and glass transition temperature for chicken meat. J Food Eng. 2002;55(1):1–8.
Sablani SS, Kasapis S, Rahman MS. Evaluating water activity and glass transition concepts for food stability. J Food Eng. 2007;72:266–71.
Sablani SS, Rahman MS, Al-Busaidi S, Guizani N, Al-Habsi N, Al-Belushi R, Soussi B. Thermal transition of King fish whole muscle, fat, fat free muscle by differential scanning calorimetry. Thermochim Acta. 2007;462:56–63.
Sunooj KV, Radhakrishna K, Johnsy George, Bawa AS. Factors influencing the calorimetric determination of glass transition temperature in foods: a case study using chicken and mutton. J Food Eng. 2009;91:347–352.
Kent M, Lees A, Nesvadba P. Detection of irradiated foods by supercooling. Food Sci Technol Today. 1994;8(2):108–9.
Rahman R, Haque AKMM, Sumar S. Physical methods for the identification of irradiated food stuffs. Nutr Food Sci. 1995;2:36–41.
Jayathilakan K, Sultana K, Radhakrishna K, Sharma GK. Effect of irradiation on differential scanning calorimetric profile of fluidized bed dried mutton. Int J Food Prop. 2012;15:202–10.
Silva VM, Park KJ, Hubinger MD. Glass transition and water sorption of spray dried mussel meat hydrolysate. XVIIth international conference on bioencapsulation, Groningen, Netherlands. 2009; 24–26.
Ohkuma C, Kawai K, Viriyarattanasak C, Mahawanich T, Tantratian S, Takai R, Suzuki T. Glass transition properties of frozen and freeze-dried surimi products: effects of sugar and moisture on the glass transition temperature. Food Hydrocolloids. 2008;22(2):255–62.
Kurozawa LE, Park KJ, Hubinger MD. Effect of maltodextrin and gum arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. J Food Eng. 2009;91(2):287–96.
Abuladze MK, Sokhadze VM, Namchevadze EN, Kiziria E, Tabatadze LV, Lejava LV, ShGogichaishvili Bakradze NB. Thermal analysis of whole bacterial cells exposed to potassium permanganate using differential scanning calorimetry: a biphasic dose-dependent response to stress. Sci World J. 2009;9:109–17.
Loomis CR, Shipley GG, Small DM. The phase behavior of hydrated cholesterol. J Lipid Res. 1979;20(4):525–35.
Thiansilakul Y, Benjakul S, Richards MP. Isolation, characterisation and stability of myoglobin from Eastern little tuna (Euthynnus affinis) dark muscle. Food Chem. 2011;124(1):254–61.
Chen LC, Lin SB, Chen HH. Thermal stability and denaturation rate of myoglobin from various species of fish. Fish Sci. 2004;70(2):293–8.
Danley RL. New heat flux DSC measurement technique. Thermochim Acta. 2002;395(1):201–8.
Lundgren CJ. High resolution TG of materials. Am Lab. 1992;24(1):49–52.
Ewing GW. Thermometric methods. In: Instrumental methods of chemical analysis, 3rd ed. Chapter 19. New York: McGraw-Hill; 1969. pp. 420–434.
Judge MD, Aberle ED. Effects of chronological age and postmortem aging on thermal shrinkage temperature of bovine intramuscular collagen. J Anim Sci. 1982;54:68.
Ellekjær MR. Assessment of maximum cooking temperatures of previously heat treated beef. Part 2: differential scanning calorimetry. J Sci Food Agric. 1992;60(2):255–61.
Funami T, Yada H, Nakao Y. Thermal and rheological properties of curdlan gel in minced pork gel. Food Hydrocoll. 1998;12(1):55–64.
Amako DE, Xiong YL. Effects of carrageenan on thermal stability of proteins from chicken thigh and breast muscles. Food Res Int. 2001;34(2):247–53.
Fukushima H, Satoh Y, Nakaya M, Ishizaki S, Watabe S. Thermal effects on fast skeletal myosins from Alaska pollock, white croaker, and rabbit in relation to gel formation. J Food Sci. 2003;68(5):1573–7.
Rasmussen D. A note about “phase diagrams” of frozen tissue. Biodynamica. 1969;10:333–9.
Simatos D, Faure M, Bonjour E, Couach M. Differential thermal analysis and differential scanning calorimetry in the study of water in foods. In: Duckworth RB, editor. Water relations of foods. London: Academic Press; 1975. p. 193–209.
Levine H, Slade L. Response to the letter by Simatos, Blond, and Le Meste on the relation between glass transition and stability of a frozen product. Cryo- Letters. 1989;10:347–70.
Brake NC, Fennema OR. Glass transition values of muscle tissue. J Food Sci. 1999;64(1):10–5.
Kurozawa LE, Park KJ, Hubinger MD. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J Food Eng. 2009;94:326–33.
Thomas LC. Interpreting unexpected events and transitions in DSC results. https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0CB0QFjAA&url=http%3A%2F%2Fwww.tainstruments.com%2Flibrary_download.aspx%3Ffile%3DTA039.PDF&ei=b_n3VOi1MJC1uQS6w4DQBQ&usg=AFQjCNE9kY1hCv8bgfIdpfUAFU9D3z1qJw&bvm=bv.87519884,d.c2E. Accessed 16 May 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamilmani, P., Pandey, M.C. Thermal analysis of meat and meat products. J Therm Anal Calorim 123, 1899–1917 (2016). https://doi.org/10.1007/s10973-015-4696-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4696-8