Abstract
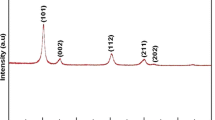
In this contribution, we report the thermal decomposition and thermo-X-ray diffraction analyses of a ZnII monomethyl terephthalate complex, [Zn(CH3O–CO–C6H4COO)2(OH2)3]·2H2O. Both XRD and temperature-dependent T-XRD patterns for the title compound in the thermal decomposition process (temperature range 30–300 °C) present diffraction peaks reminiscent of ordered, crystalline structure of the starting complex [Zn(CH3O–CO–C6H4COO)2(OH2)3]·2H2O. Crystallization and coordination water molecules of the title complex are eliminated in successive steps, and then the anhydrous complex decomposes to ZnO (the total experimental mass loss of 84.80 % versus the theoretical mass loss, 84.23 %). The formation of ZnO of wurtzite structure (hexagonal phase, space group P63 mc) as spherical nanoparticles with average size of 58 nm has been confirmed by XRD, SEM and EDX analyses performed on the final product.






Similar content being viewed by others
References
Rao CNR, Natarajan S, Vaidhyanathan R. Angew Chem Int Ed. 2004;43:1466–96.
Cui Y, Evans OR, Ngo HL, White PS, Lin W. Angew Chem Int Ed. 2002;41:1159–62.
Baca SG. IJPAC-Int Res J Pure Appl Chem. 2012;2(1):1–24.
Kitagawa S, Kitaura R, Noro S-i. Angew Chem Int Ed. 2004;43:2334–75.
Mori W, Sato T, Ohmura T, Kato CN, Takei TJ. Solid State Chem. 2005;178:2555–73.
Mori W, Takamizawa S. J. Solid State Chem. 2000;152(10):120–9.
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM. Nature. 1999;402:276–9.
Hawxwell SM, Adams H, Brammer L. Acta Cryst. 2006;B62:808–14.
Acheson RJ, Galwey AK. J. Chem. Soc. A 1967;1174–1178.
Şerb M-D, Wang Y, Dumitru F, Englert U. Acta Cryst. 2011;E67:m475–6.
Li H, Eddaoudi M, Groy TL, Yaghi OM. J Am Chem Soc. 1998;120:8571–2.
Clausen HF, Poulsen RD, Bond AD, Chevallier M-AS, Iversen BB. J Solid State Chem. 2005;178:3342–51.
Sun J, Zhou Y, Fang Q, Chen Z, Weng L, Zhu G, Qiu S, Zhao D. Inorg Chem. 2006;45:8677–84.
Yin P-X, Zhang J, Li Z-J, Qin Y-Y, Cheng J-K, Yao Y-G. Inorg Chem Commun. 2008;11:134–7.
Carton A, Mesbah A, Aranda L, Rabu P, Francois M. Solid State Sci. 2009;11:818–23.
Roy S, Sarkar BN, Bhar K, Satapathi S, Mitra P, Ghosh BK. J Mol Str. 2013;1037:160–9.
Donald Kirkbright Black, US Patent 4058663, 1977.
Tranchemontagne DJ, Mendoza-Cortés JL, O’Keeffe M, Yaghi OM. Chem Soc Rev. 2009;38:1257–83.
Spek AL. Acta Cryst. 2009;D65:148–55.
Brzyska W, Wańczowska-Fonfara D. J Therm Anal Calorim. 1989;35(3):727–33.
Carp O, Patron L, Segal E. Rev Roum Chim. 2006;51(1):5–12.
Findoráková L, Györyová K, Hudecová D, Mudroñová D, Kovářová J, Homzová K, Nour El-Dien FA. J Therm Anal Calorim. 2013;111:1771–81.
Bujdošová Z, Györyová K, Kovářová J, Hudecová D, Halás L. J Therm Anal Calorim. 2009;98:151–9.
Brzyska W, Ozga W. J Therm Anal Calorim. 2002;67:623–9.
Skoršepa J, Godočíková E, Černák J. J Therm Anal Calorim. 2004;75:773–80.
Kurpiel-Gorgol R, Brzyska W. J Therm Anal Calorim. 2003;71:539–48.
Krajníková A, Györyová K, Kovářová J, Hudecová D, Hubáčková J, Nour El-Dien F, Koman M. J Therm Anal Calorim. 2012;110(1):177–85.
Morkoç H, Özgur Ü, Zinc oxide: fundamentals, materials and device technology. Wiley. KGaA, Weinheim: GmbH & Co; 2009. ISBN 978-3-527-40813-9.
Wang ZL. J Phys Condens Matter. 2004;16:R829.
Acknowledgements
Authors recognize financial support from the European Social Fund through POSDRU/89/1.5/S/54785 project: Postdoctoral Program for Advanced Research in the field of nanomaterials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Şerb, MD., Müller, P., Truşcă, R. et al. Study of thermal decomposition of a zinc(II) monomethyl terephthalate complex, [Zn(CH3O–CO–C6H4COO)2(OH2)3]·2H2O. J Therm Anal Calorim 121, 691–695 (2015). https://doi.org/10.1007/s10973-015-4629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4629-6