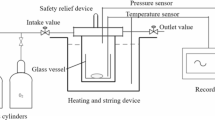
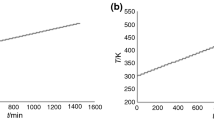
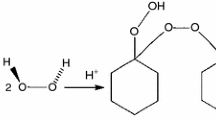
Abstract
The influence of a degraded solvent on Grignard reagent formation was investigated in terms of heat release behaviour. Since degraded solvent is supposed to contain peroxide by oxidation with air as well as water from atmosphere, peroxide in this study was intentionally produced by the storage of tetrahydrofuran in a pressurized oxygen atmosphere and the induction periods were measured. The induction period which appears prior to the main exothermic reaction increased with increasing amounts of peroxide and water content, respectively. However, there was a difference in heat release behaviour; the reaction including peroxide showed a delay in the heat release and two exothermic peaks, while the solvent that included water simply showed a delay in the start of the reaction. γ-Butyrolactone was found to be a product derived from peroxide, by component analysis of the oxidized solvent. γ-Butyrolactone caused an induction period when it was added to the solvent. This work suggests a deteriorated solvent can lead to inappropriate handling which cause a runaway reaction and gives insight to the chemical industry that manages the risk of potentially hazardous chemical reactions.














Similar content being viewed by others
References
Bou-Diab L, Fierz H. Autocatalytic decomposition reactions, hazards and detection. J Hazard Mater. 2002;93:137–46.
Gustin JL. Thermal stability screening and reaction calorimetry Application to runaway reaction hazard assessment and process safety management. J Loss Prevent Proc. 1993;6:275–91.
Serra E, Nomen R, Sempere J. Maximum temperature attainable by runaway of synthesis reaction in semi-batch processes. J Loss Prevent Proc. 1997;10:211–5.
Kumasaki M. The solvent effects on Grignard reaction, IChemE Symposium Series No. 53; 2007.
Am Ende DJ, Clifford PJ, DeAntonis DM, SantaMaria C, Brenek SJ. Preparation of Grignard reagents: FTIR and calorimetric investigation for safe scale-up. Org Process Res Dev. 1999;3:319–29.
Tanaka K, Kumasaki M, Miyake A. Influence of Mg surface layer for induction period of Grignard reagent formation. J Therm Anal Calorim. 2013;113:1395–401.
Garst JF, Soriaga MP. Grignard reagent formation. Coord Chem Rev. 2004;248:623–52.
Kanoufi F, Combellas C, Hazimeh H, Mattalia J-M, Marchi-Delapierre C, Chanon M. Alkyl halides reactions with cathodes or with magnesium. Grignard reagent studied with radical clocks. What is the step competing with the isomerisation of the intermediate radical? J Phys Org Chem. 2006;19:847–66.
Tilstam U, Weinmann H. Activation of Mg metal for safe formation of grignard reagents on plant scale. Org Process Res Dev. 2003;6:906–10.
Reichardt C, Welton T. Solvents and solvent effects in organic chemistry. Weinheim: Wiley-VCH; 2011.
Dasler W, Bauer CD. Removal of peroxides from organic solvents. Ind Eng Chem. 1946;18:52–4.
Hamstead AC. Destroying peroxides of isopropyl ether. Ind Eng Chem. 1964;56:37–42.
Armarego WLF, Chai C. Purification of laboratory chemicals. USA: Butterworth-Heinemann; 2013.
Kito H, Fujiwara S, Kumasaki M, Miyake A. Assessment of autoxidative resistance for organic solvent by pressure monitoring test. Int J Saf. 2010;9:43–6.
Mitchell J. Organic analysis, vol. 4. New York: Interscience; 1960. p. 1–64.
Hawkins EGE. Organic peroxides. London: E.&FF. Spon; 1961. p. 332.
Johnson RM, Siddiqi IW. The determination of organic peroxides. New York: Pergamon Press; 1970.
Swern D. Organic peroxides, vol. 1. NewYork: Wiley-Interscience; 1969. p. 497.
Kumasaki M, Fujimoto Y, Ando T. Calorimetric behaviors of hydroxylamine and its salts caused by Fe(III). J Loss Prevent Proc. 2003;16:507–12.
Kumasaki M. An explosion of a tank car carrying waste hydrogen peroxide. J Loss Prevent Proc. 2006;19:307–11.
Kumasaki M. Calorimetric study on the decomposition of hydroxylamine in the presence of transition metals. J Hazard Mater. 2004;115:57–62.
Kowhakul W, Kumasaki M, Arai M, Tamura M. Calorimetric behaviors of N2H4 by DSC and superCRC. J Loss Prevent Proc. 2006;19:452–8.
Kryk H, Hessel G, Schmitt W, Tefera N. Safety aspects of the process control of Grignard reactions. Chem Eng Sci. 2007;62:5198–200.
Shurvell HF, Southby MC. Infrared and Raman spectra of tetrahydrofuran hydroperoxide. Vib Spectrosc. 1997;15:137–46.
McDermott DP. Vibrational assignments and normal-coordinate analyses of γ-butyrolactone and 2-pyrrolidinones. J Phys Chem. 1986;90:2569–74.
Rein H, Criegee R. Ueber das Tetrahydrofuran-peroxyd. Angew Chem. 1950;62:120.
Vozza JF. Products of the reaction between gamma-butyrolactone and phenylmagnesium bromide. J Org Chem. 1959;24:720–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumasaki, M., Tanaka, K. & Otsuka, T. Influence of deteriorated solvent on induction period of Grignard reagent formation. J Therm Anal Calorim 120, 633–639 (2015). https://doi.org/10.1007/s10973-015-4436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4436-0