Abstract
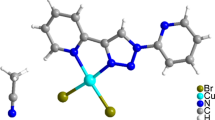
A copper(II) Schiff-base complex [CuL·2MeOH·H2O] was prepared by reaction of the ligand [H2L = N, N′-o-phenylene-bis(3-methoxysalicylideneimine)] and Cu(OAc)2·H2O in methanol at 328.15 K. X-ray diffraction analysis revealed that the title complex was crystallized in the triclinic crystal system with space group P–1, in which copper(II) ion was located in the N2O2 ligand binding sites. According to two designed thermochemical cycles, the dissolution enthalpies of relevant substances of two reactions in the calorimetric solvents were determined by a solution-reaction isoperibol calorimeter, respectively. Combined with some other auxiliary thermodynamic data, the standard molar enthalpies of formation of the ligand and the complex were calculated to be: \( \Delta_{\text{f}} H_{\text{m}}^{\theta } \)[H2L(s), 298.15 K] = −(384.2 ± 9.10) kJ mol−1 and \( \Delta_{\text{f}} H_{\text{m}}^{\theta } \)[CuL·2MeOH·H2O (s), 298.15 K] = −(1072.6 ± 9.2) kJ mol−1.






Similar content being viewed by others
References
Yamada S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord Chem Rev. 1999;192:537–55.
Liu XL, Shang XM, Tang T, Hu NH, Pei FK, Cui DM, Chen XS, Jing XB. A chiral lanthanide alkyl complexes bearing N,O multidentate ligands. Synthesis and catalysis of highly heteroselective ring-opening polymerization of rac-lactide. Organometallics. 2007;26:2747–57.
Agrawal R, Sharma J, Nandani D, Batra A, Singh Y. Triphenylarsenic(V) and antimony(V) derivatives of multidentate Schiff bases: synthesis, characterization, and antimicrobial activities. J Coord Chem. 2011;64:554–63.
Ungur L, Thewissen M, Costes JP, Wernsdorfer W, Chibotaru LF. Interplay of strongly anisotropic metal ions in magnetic blocking of complexes. Inorg Chem. 2013;52:6328–37.
Patel MN, Patel CR, Joshi HN. Synthesis, characterization and biological studies of mononuclear copper(II) complexes with ciprofloxacin and N,O donor ligands. Inorg Chem Commun. 2013;27:51–5.
Wilson TD, Yu Y, Lu Y. Understanding copper-thiolate containing electron transfer centers by incorporation of unnatural amino acids and the CuA center into the type 1 copper protein azurin. Coord Chem Rev. 2013;257:260–76.
Ghosh K, Mohan V, Kumar P, Singh UP. DNA binding, nuclease and superoxide scavenging activity studies on mononuclear cobalt complexes derived from tridentate ligands. Polyhedron. 2013;49:167–76.
Feltham HLC, Clerac R, Powell AK, Brooker S. A tetranuclear, macrocyclic 3d–4f complex showing single-molecule magnet behavior. Inorg Chem. 2011;50:4232–4.
Lu XQ, Feng WX, Hui YN, Wei T, Song JR, Zhao SS, Wong WY, Wong WK, Jones RA. Near-infrared luminescent, neutral, cyclic Zn2Ln2 (Ln = Nd, Yb, and Er) complexes from asymmetric salen type Schiff base ligands. Eur J Inorg Chem. 2010;18:2714–22.
Feng X, Wang L, Zhao J, Wang J, Weng NS, Liu B, Shi X. Series of anion-directed lanthanide-rigid-flexible frameworks: syntheses, structures, luminescence and magnetic properties. Cryst Eng Comm. 2010;12:774–83.
Vigato PA, Tamburini S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord Chem Rev. 2004;248:1717–2128.
Yu HG, Liu Y, Tan ZC, Dong JX, Zou TJ, Huang XM, Qu SS. A reaction-isoperibol calorimeter and standard-molar enthalpies of formation of Ln(hq)2Ac (Ln = La, Pr). Thermochim Acta. 2003;401:217–24.
Gu HW, Xiao SX, Xiao HY, Xiao Y, Li AT, Hu XL, Li QG. Synthesis, characterization, and thermodynamic properties of the rare earth coordination complex [Sm(C6H4NO2)2·C9H6NO]. J Chem Eng Data. 2012;51:4797–803.
Li Q, Yan P, Chen P, Hou G, Li G. Salen type sandwich triple-decker tri- and di- nuclear lanthanide complexes. J Inorg Organomet Polym. 2012;22:1174–81.
Rychly R, Pekarek V. The use of potassium chloride and tris(hydroxymethyl)aminomethane as standard substances for solution calorimetry. J Chem Thermodyn. 1977;9:391–6.
Cox JD, Wagman DD, Medvedev VA. CODATA key values for thermodynamics. Chapter 1, New York: Hemisphere Publishing Corp.; 1984.
Weast RC. CRC handbook of chemistry and physics. 69th ed., Boca Raton: CRC Press Inc; 1988/1989. p. D-121.
Li X, Jiang JH, Gu HW, Xiao SX, Li CH, Ye LJ, Li X, Li QG, Xu F, Sun LX. Calorimetric measurement of the standard molar enthalpies of formation of o-vanillin and trimethoprim. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-4184-6.
Contineanu I, Wagner L, Stanescu L, Marchidan DI. Combustion and formation enthalpies of o-phenylenediamine, urea and 2-benzimidazolone. Rev Roum Chim. 1982;27:205–9.
Kunyavskaya SG, Karyakin NV, Krylova GP, Chernova VI, Rabinovich IB. Heat capacity and thermodynamic properties of iosmeric phenylenediamines. Tr Khim Khim Tekhnol. 1973;1:58–9.
Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21273190), Science and Technology Plan Projects of Hunan Province, China (Nos. 2014FJ3011, 2013FJ3033), the Hunan Provincial Natural Science Foundation of China (No. 11JJ3019), the College Student Innovative Experimental Project of Xiangnan University (The Design for Drainage-oil Test Pen), the Hunan Provincial Colleges and Universities “twelve-five planning” Professional Comprehensive Reform Pilot Project (No. 2012-266), the Construction Project of Hunan Provincial Colleges and Universities Teaching Practice (No. 2013-295), the Scientific Research Foundation of Xiangnan University (Nos. 2013YJ36, 2013YJ37, 2013YJ38), and the Construct Program of the Key Discipline in Hunan Province for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, CH., Jiang, JH., Yang, P. et al. Preparation, structure, and thermochemical properties of a copper(II) Schiff-base complex. J Therm Anal Calorim 119, 1285–1292 (2015). https://doi.org/10.1007/s10973-014-4232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4232-2