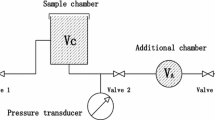
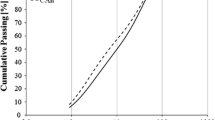
Abstract
This article presents the investigations of the progress of conversion process of calcium aluminate hydrates formed during hydration of calcium aluminate cement at various temperature conditions occurring over time by thermal analysis method. Moreover, the differences of microstructure were also confirmed by SEM/EDS studies and X-ray diffraction analysis. On the basis of the obtained results, it is concluded that thermal analysis method is a very attractive and useful way to identify the structure of hydrated calcium aluminate cement matrix and allows estimating the degree of the conversion at different times of various process conditions. The conversion process of metastable calcium aluminate hydrates into stable hydrogarnet and gibbsite is strictly temperature dependent and could be completed at different times. Acceleration of the conversion is caused not only by the increasing external temperature of storage, but also the temperature inside the sample is very important. The self-heating, which could be strong in large sample, and occurring during first few hours of hydration of calcium aluminate cement, initiates the transformation.





Similar content being viewed by others
References
Bensted J. Scientific aspects of high alumina cement. Cem Lime Concr. 2004;3:109–33.
Kurdowski W. Chemistry of cement and concrete. Warsaw: PWN/Polski Cement Editor; 2010 (in Polish).
Pacewska B, Nowacka M, Wilińska I, Kubissa W, Antonovic V. Studies on the influence of spent FCC catalyst on hydration of calcium aluminate cements at ambient temperature. J Therm Anal Calorim. 2011;105(1):129–40.
Hidalgo A, Garcia JL, Alonso MC, Fernandez L, Andrade C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J Therm Anal Calorim. 2009;96(2):335–45.
Ramachandran VS, Paroli RM, Beaudoin JJ, Delgado AH. Handbook of thermal analysis of construction materials. New York: Noyes Publications, William Andrew Publishing; 2002.
Neville AM. Properties of concrete. Cracow: Polski cement Editor 2012 (in Polish).
Standard EN 14647:2007. Calcium aluminate cement—Composition, specifications and conformity criteria.
Bensted J, Smith JR. High alumina cement—some important aspects. Cem Lime Concr. 2011;4:215–23.
Kontori E, Perraki T, Tsivilis S, Kakali G. Zeolite blended cements: evaluation of their hydration rate by means of thermal analysis. J Therm Anal Calorim. 2009;96:993–8.
Palou MT, Bágel’ L, Živica V, Kuliffayová M, Ifka T. Hydration of high alumina cement–silica fume composite with addition of Portland cement or sodium polyphosphate under hydrothermal treatment. J Therm Anal Calorim. 2013;113:385–94.
Wongkeo W, Thongsanitgarn P, Chindaprasirt P, Chaipanich A. Thermogravimetry of ternary cement blends Effect of different curing methods. J Therm Anal Calorim. 2013;113:1079–90.
Alarcon-Ruiz L, Platret G, Massieu E, Ehrlacher A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem Concr Res. 2005;35:609–13.
Pane I, Hansen W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem Concr Res. 2005;35:1155–64.
Darquennes A, Espion B, Staquet S. How to assess the hydration of slag cement concretes. Constr Build Mater. 2013;40:1012–20.
Information of Górkal cement producer, published on a webpage: www.gorka.com.pl.
Ukrainczyk N, Matusinovic T, Kurajica S, Zimmermannb B, Sipusic J. Dehydration of a layered double hydroxide—C2AH8. Thermochim Acta. 2007;464:7–15.
Pacewska B, Nowacka M, Aleknevičius M, Antonovič V. Early hydration of calcium aluminate cement blended with spent FCC catalyst at two temperatures. Proc Eng. 2013;57:844–50.
Gosselin Ch, Gallucci E, Scrivener K. Influence of self heating and Li2SO4 addition on the microstructural development of calcium aluminate cement. Cem Concr Res. 2010;40:1555–70.
Lothenbach B, Pelletier-Chaignat L, Winnefeld F. Stability in the system CaO–Al2O3–H2O. Cem Concr Res. 2012;42:1621–34.
Taylor HFW. Cement chemistry. 2nd ed. London: Thomas Telford; 1998.
Rashid S, Barnes P, Bensted J, Turrillas X. Conversion of calcium aluminate cement hydrates re-examined with synchrotron energy-dispersive diffraction. J Mater Sci Lett. 1994;13:1232–4.
Acknowledgements
The authors would like to thank M.Sc. Barbara Trybalska for the performance of the SEM/EDS analysis, and M.Sc. Sławomir Pilarczyk for his kind help with XRD measurements. This publication has been partly co-financed from the European Union funds granted by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacewska, B., Nowacka, M. Studies of conversion progress of calcium aluminate cement hydrates by thermal analysis method. J Therm Anal Calorim 117, 653–660 (2014). https://doi.org/10.1007/s10973-014-3804-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3804-5