Abstract
Vitrification is the most effective method of the hazardous waste immobilization. Toxic elements are incorporated into glass structure. Iron phosphate glasses are presently being considered as a matrix for storage of the radioactive waste which cannot be vitrified using a conventional borosilicate glass. Influence of Na2SO4 as one of the components such the waste on thermal properties and crystallization ability of iron phosphate waste glass was studied. It was observed that Na2SO4 decreases transformation temperature and increases ΔC p. The glass characteristic temperatures, glass crystallization ability, and crystallizing phases were determined. Na2SO4 increases the glass crystallization ability which could be related with ΔC p heat capacity accompanying glass transition changes. The glass internal structure rebuilding, accompanying the sodium content increase, is considered. It is shown that ΔC p is a suitable, structure-sensitive glass crystallization ability, parameter.
Similar content being viewed by others
Introduction
Vitrification is the most effective methods of the hazardous waste immobilization. Toxic elements are incorporated into glass structure. Borosilicate glasses are of common use in nuclear waste immobilization procedure. Lately, glasses from Fe2O3–P2O5 system are of the great interest, for scientific reason and because they are considered as a high-capacity matrix for storage of radioactive waste [1, 2]. The worldwide-used borosilicate glasses for nuclear waste vitrification are not suitable for immobilization of high-content molybdenum, chromium, or salt waste because of the low solubility of these compounds in the borosilicate glass Therefore, it is necessary to look for an alternate vitrification matrix. The next choice is the phosphate glasses. However, the most of phosphate glasses have a low chemical durability, but iron as a glass component significantly increases it. Among other phosphate glasses the highest chemical durability is reported for 60P2O5–40Fe2O3 (Fe/P = 0.67) glass. All of them have O/P ratio over 0.3 and are classified as polyphosphate glasses [3]. It indicates that iron strengthens the chemical bonds in glass structure making their properties comparable or even better than borosilicate glasses [1, 2].
The iron phosphate glasses, besides their excellent chemical durability, have the melting temperature about 100–200 K lower than borosilicate glass, and due to the high fluidity of the melts, their homogenization time is about 1.5–3 h shorter [4]. It is way they can be suitable for vitrifying waste containing significant quantities of volatile elements such as Cs. Moreover, iron phosphate glasses exhibit particularly high thermal stability in terms of the low crystallization tendency and can be produced in big quantities without danger of crystallization matrix. Depending on the composition and heating schedule, glassy materials could be obtained but often with crystalline particulate inclusions [1].
Use of the iron phosphate glasses for vitrification of waste which contains: relatively high concentration of actinide elements, high concentration of sodium, cesium sulfate, chloride-containing waste, chloride waste from pyrochemical reprocessing of Pu metal, waste containing various metals, radioactive ceramics, polymers, and carbon is now being considered [1, 5].
Taking all of these into account in the present paper, the influence of Na2SO4 as one of the salty waste components on the thermal properties, especially glass stability against crystallization of P2O5–Fe2O3 of constant Fe/P = 0.6 glass was studied. The structural aspects of the high capacity of salt waste incorporation are being considered as well.
Experimental
Glass containing 37 mol% Fe2O3 and 63 mol% P2O5 was prepared from chemically pure NH4H2PO4 and Fe2O3. Batches were melted for 2 h at 1,373 K in Al2O3 crucible in an electric furnace with the furnace atmosphere as close to natural as possible. The melt was vitrified by casting onto steel plate and crushed into 0.3- to 0.1-mm grain size. The obtained glass frit was mixed with Na2SO4 (used as waste simulator) and remelted at 1,373 K for 2 h. Na2SO4 to the glass ratio was x = 0, 10, 20, 30, and 50 mass%. Samples were designated as PFS0, PFS10, PFS20, PFS30, and PFS50, respectively (Table 1).
Glass transformation temperature T g at the half of the heat capacity step on DSC curve, crystallization T C as the onset of the first crystallization peak, and melting T M as the onset of the first melting peak temperatures were measured by differential scanning calorimetry (DSC) method at the heating rate of 10 K min−1. Measurements were carried out using Perkin Elmer DTA/DSC/TG—seven heat flow differential scanning calorimeter. Pure Al and Au were used for temperature and heat calibration. Value of specific heat capacity change ΔC p (J g−1 K−1), accompanying glass transformation, was determined using Perkin Elmer Pyris Thermal Analysis Software Library program.
Crystallization of the glasses was checked by heating the powdered samples by 2 h at T c temperature of exothermic DSC peak. Phase composition of the samples was investigated by X-ray diffractometry (XRD) using DRON 1.5 apparatus, and Cu Kα radiation and the measured spectra were analyzed using X’Pert HighScore Plus software. The obtained spectra were fitted using Rietveld method, and semi-quantitative analysis of crystallizing phases was done.
Glass density was measured by weightening of bulk glass samples in air and in water.
Results
The effect of addition of the Na2SO4 to the iron phosphate glass on its thermochemical properties is demonstrated by the DSC curves. An exemplary DSC curve for the base glass and the samples containing 20 and 50 mass% of sodium sulfate is presented in Fig. 1. All the samples show the transformation step which is increasing with Na2SO4 content and follow to the increase of the value of molar heat capacity change ΔC p accompanying the glass transformation. Just behind, there appears an exothermic effect of crystallization, small and diffuse in the case of the base glass and stronger and stronger when sodium is added. After crystallization effect, melting of the glass occurs. The similar curves are observed for the rest of the measured samples. The transformation, crystallization, and melting temperatures of the investigated materials are presented in Table 2.
Influence of the Na2SO4 content on transformation temperature T g and the molar heat capacity ΔC p accompanying the glass transformation is presented in Fig. 2. The T g temperature is reduced with x increase; then, a kind of plateau appears (Fig. 2), and it persists almost constant upto about x = 30 than is dropping down again. The ΔC p value is growing continuously with the sodium sulfate up to 30 mol% content (Fig. 2).
One of the important parameters in the case of waste vitrification is the thermal stability of the vitrified product. The glass stability of the investigated materials was evaluated using Hruby criterion. According to [6], the higher the K H value the greater its stability against crystallization. The K H value was evaluated according to the formulae:
The obtained K H parameters are summarized in Table 2 and Fig. 3.
All of the investigated glasses except glass PFS50 evidenced good thermal stability comparable with conventional silicate glasses for which K H value varied from 0.14 to about 1.3 [7–9]. The increasing Na2SO4 addition causes decrease of the K H parameter which means lowering the glass stability against crystallization and at the same ability of glass to vitrify on cooling [9].
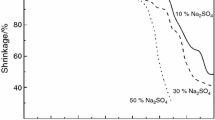
Enthalpy of crystallization calculated from DSC curves’ exothermic peak area was taken as an arbitrary measure of the glass crystallization degree. Its value is growing up rapidly with Na2SO4 content but above x = 30 mol% is falling down (Fig. 4). Accordingly, the shape of DSC peak is changing (Fig. 1). It is more and more narrow and sharp, which indicates the change of crystallization kinetics.
To quantify this observation, the ratio ΔH/Δt was taken as crystallization factor. It is changing at the range of 20–30 mol% Na2SO4 content (Fig. 5). This suggests the change of the crystallization mechanism above the Na2SO4 20 mol% content.
The XRD patterns of the compounds crystallizing in PFS0, PFS20, and PFS50 glasses at Tc temperature indicated by the DSC are presented in Fig. 6 and Table 3. The main crystalline phases formed in the base glass (PFS0) are iron phosphates FePO4 and Fe II2 (P2O7)2. Addition of sodium besides iron phosphate results in the formation of the sodium containing compounds as NaFeIIIP2O7. Further increasing of sodium concentration leads to crystallization of Na3Fe III2 (PO4)3 which crystallizes at cost of the iron phosphates. For the glass PFS50, there is no evidence of crystallization of FePO4 or Fe2 3+Fe2+(P2O7)2 but formation of sodium-rich phases, like Na3Fe2(PO4)3 and Na7Fe3(P2O7)4, is detected. Generally, in iron phosphate glasses, composition of the crystallizing compounds depends on the glass composition, heat treatment and its atmosphere, and the Fe2+/Fe3+ ratio. Phases formed in the glasses investigated here are similar to those observed in the case of crystallization cesium iron phosphate glasses [10, 11].
The Na2SO4 incorporation leads to growth of Na2O content and increases the change of the specific heat capacity ΔC p accompanying the glass transformation (Fig. 2b). As was mentioned previously [12, 13], ΔC p value can be the indicator of a degree of the structural changes accompanying the transformation, like number and strength of the broken bonds and rearrangement of structure constituents.
Results of the measurements proved the relation between ΔC p and the K H values which could describe glass stability against crystallization (Fig. 7). As can be clearly visible, the higher the ΔC p value means the lower the glass stability and in the same way the lower the K H parameter. This proves that the ΔC p value obtained by DSC method, could be used also as glass stability against crystallization parameter. The ΔC p value is related to the change of entropy accompanying glass transition and at the same as the structure rebuilding degree indicator [14].
The glass densities and molecular volumes accompanying them are presented in Fig. 8. As can be clearly seen, the glass density increases up to x = 20 and for higher Na2SO4 content is decreasing. The molar volume behaves in the opposite way.
Discussion
Iron belongs to the group of metallic elements B, Al, and Fe which in their three valent state possess an ability to form specific phosphate glasses of composition corresponding to orthophosphates BPO4, AlPO4, and FePO4. They distinguish the higher chemical durability and thermal stability, as compared to glasses containing these elements but having lower metal (Me) to phosphorous Me/P ratio. Structure and properties of the orthophosphate glasses are not recognized good enough, specially the iron phosphate glasses.
The most information can be found about AlPO4 glass and its crystalline analog berlinite. Berlinite structure is close to the structure of quartz (SiO2) framework silicate, but instead of [SiO4] tetrahedra it is built of [AlO4][PO4] groups, geometrically, and crystallochemically equivalent to [SiO4][SiO4] pair. Similarity is so close that berlinite like quartz has tridymite- and cristobalite-like polymorphic modifications. It is also generally supposed that AlPO4 glass has the vitreous SiO2-like structure. Silica glass (v-SiO2) structure accepts some quantities of AlPO4 and mixed network glass, chemically durable and high-temperature resistant is formed.
The BPO4 exhibits analogous similarity to SiO2 structure and dissolves in vitreous silica. As it was demonstrated earlier, BPO4–SiO2 glass dissolves considerable quantities of AlPO4 and three component AlPO4–BPO4–SiO2 glass with polymeric mixed network is formed [15]. NMR studies of this glass proved that in its structure, 2/3 Al3+ has coordination number (CN) 4 and B3+ cation possesses the same coordination. This indicates that structure of the glass consists of [AlO4], [BO4], and [SiO4] tetrahedra, joined together by oxide bridges. It means that all of them play the same function in the structure of glass, as network-formers. Addition of Na2O in the quantities higher than 10 mol% changes the CN of part of three valent cations in structure, and [AlO6] and [BO3] polyhedra appear. Their number increases with Na2O content [15].
In the chain structure of metaphosphate Na2O–Al2O3–P2O5 glasses, Al3+ has CN 6 and together with Na+ alternatively saturates the charge of the non-bridging oxygen in the phosphate chains. Similar Al and Na arrangements appear in pyrophosphate glasses from this system. In the orthophosphate glass structure [PO4], tetrahedron is saturated by two Al3+ ions with the CN 4 and one with CN 6 and plus one cation Na+. With the Al2O3 content increase, the content of alumina in coordination 4 and orthophosphate [PO4] groups increase accordingly [16]. When the ratio Al2O3/P2O5 exceeds 0.63, glass network is made up mainly by the [AlO4] and [PO4] tetrahedra, joined each other by the bridging oxygen’s—mixed alumino-phosphate network [17].
Iron orthophosphate FePO4 possesses many similarities to berlinite, and it has also iron tridymite and iron cristobalite modifications, which indicates the structural similarity of both of the compounds.
However, according to investigations of local iron environment in phosphate glass 40Fe2O3–60P2O5 (mol%) melted at varying temperatures and under different atmospheric conditions, it contains both FeIII and FeII ions [18]. The average iron–oxygen coordination is 4–5 and iron ions have mixed tetrahedral–octahedral coordination and Fe3+ ions occupy two distinct sites in glass structure.
Existence of FeO5 instead of FeO6 polyhedra for FeII and FeO6 octahedra for Fe3+ in the glass of ultraphosphate composition ware was suggested in [19]. On the other hand, Mössbauer spectroscopy study of the SiO2–P2O5–Al2O3–Fe2O3–CaO containing different, up to 14.8, mass% of Fe2O3 [22] indicates that coordination number of iron cations depends on Fe2+ to the total Fe content ratio. For glasses synthesized in oxidizing condition, iron in both Fe2+ and Fe3+ state exists predominantly in tetrahedral coordination. In more reduced conditions, glass abundance of tetrahedrally coordinated Fe3+ decreases linearly with increasing Fe2+ content.
Basic glass investigated here contains 63 mol% P2O5 and 37 mol% Fe2O3 and Fe/P ratio is equal to 0.6, which corresponds the polyphosphate compounds which have chain and ring phosphate groups in the structure. To saturate free phosphate charges in such a structure, the valence of part of Fe3+ has to be reduced to Fe2+. High melting temperature of the glass and excess of phosphate groups in melts promote the Fe3+ reduction. To completely saturate the phosphate charges, the melt should contain 27 mol% orthophosphate FeIII[PO4] groups and 73 mol% of chains of FeII[P2O7].
The XRD pattern demonstrated that both of these phosphates crystallize in the glass, heat-treated (Table 3), and quantitative proportions of them are close to calculated above. As it was demonstrated in [20, 21], between T g and liquid temperature, at the viscoelastic state of glass, its crystallization proceeds by successive ordering and diffusional growth of local groups of atoms, forming middle range order clusters. The basic glass crystallization was carried out in DSC-measured temperature which is close to T g and much lower than melting temperature (Table 1). This suggests that basic glass structure contains polymerized Fe[PO4] groups, phosphate chains, and/or pyrophosphate pairs of phosphate tetrahedral [P2O7]4−.
According to XRD results, Na2SO4 incorporation into the basic glass changes its physical properties, which is accompanied by internal structure rebuilding. This is evidenced by the composition of compounds crystallizing in glass. Sodium oxide increases the oxygen content in structure of glass, promoting oxidation of Fe2+ and Fe3+ content increase. Moreover, Na+ participates in the saturation of phosphate charges and Na, Fe-phosphates crystallize in glass. In 10 mol% Na2SO4-containing glass, this is NaFeP2O7 and its number is growing up. When it reaches 50 mol%, compounds NaFeP2O7, Na3Fe2(PO4)3, and Na7Fe3(P2O7)4 are crystallizing (Table 3).
Addition of Na2O to the basic glass increases the metallic cations to phosphorus Na + Fe/P ratio from 0.6 in basic glass to 2.1 for PFS50 sample. Change in structure of the glasses is a result of this as well. In oxide glasses, Na+ breaks the oxide bridges, disintegrating the network. Part of Fe–O–P bonds is broken and substituted by Na–PO4 bonds, and number of this substitution is growing with sodium content. Formulas of compounds crystallizing in glasses (Table 3) are the argument for this. The substitution degree expressed by Na/FeIII ratio is growing in them, it needs, in parallel progressive decomposition of phosphate network, into pyrophosphate elements and finally, when Na + Fe/P exceeds 2.0, into separate [PO4] tetrahedra (PFS50 glass). The latest glass has chemical formula 1.6Na2O·0.6Fe2O3·P2O5, which means that its structure consists of pyro- and ortho-phosphate groups surrounded by Na+ and Fe3+ cations. Accordingly, molecular volume (V) of glass changes with Na2O content increase. At the beginning, its value is going down which is demonstrated by the structure density increase and next is growing up (Fig. 8). Content of 20 mol% of Na2SO4 and Na + Fe/P > 1 is the turning point of molecular volume vs sodium oxide (x) content relation. Structure densification can be explained as an effect of the increase of Fe3+ content being the effect of oxidizing action of Na2O and stabilizing three valent iron existence. Moreover, Na+ is breaking part of P–O–P bridges and small pyrophosphate groups, surrounded by Fe3+ plus Na+ are formed. Smaller network-forming P2O5 groups surrounded by Na+ and joined with them by undirectional ionic bonds make the structure flexible. This leads to possibility of the glass structure components to change their positions according to the chemical minimum free energy rule.
Further, Na2O content increases, above the turning point, when Na + Fe/P ratio exceeds 1, resulting in molar volume increase (Fig. 8). This can be explained as disintegration of phosphate network groups, up to separate [PO4] tetrahedral formation. Mechanism of the structure rebuilding, proposed here, has support in the chemical composition of compounds crystallizing in glass (Table 3). It explains also the change of thermal properties of glass as Na2SO4 content dependent (x).
The Na2SO4 incorporation increases the change of the specific heat capacity ΔC p accompanying the glass transformation (Fig. 2b). As was mentioned previously [12, 13], ΔC p value can be the indicator of configuration entropy increase as dependent on degree of the structure organization changes, accompanying the transformation, like number of the broken bonds and forms of existence and arrangement of constituents of structure. As it was mentioned above, sodium into glass structure breaks network oxide bridges, reducing their number and separating network into smaller, at the same more mobile species. Accordingly in structure, the number of undirectional ionic Na–O bonds increases. All of these make the glass structure more flexible and easier to reorganize which can be deeper and deeper with x value increase, and explains ΔC p increase, accompanying T g decrease (Fig. 2a).
Curve of the T g vs x value relation is dropping down rapidly at the beginning but near the turning point x = 20 mol%, it stabilizes and characteristic plateau appears (Fig. 2a). It is accompanied by change of slope of the curve of ΔC p of glass transformation (Fig. 2b). This indicates that first stage of structure rebuilding is a source of the relatively high change of heat capacity, suggesting the deep structure reorganization. It can be a consequence of the iron oxidation and number of Fe–O–P bridges linking phosphate group increase and structure of glass stabilization. Above the turning point at Na2O 20 mol%, further increase of ΔC p is incomparably smaller, which means the smaller differences in the elements of structure composition and distribution, as it was suggested above. Increasing molar volume value together with diminishing number of Fe as phosphate group joining component, and increasing Na in composition of the crystallizing compounds, suggests diminishing number of the Fe–O–P cross-linking bridges and weakening structure of glass. Properly, glass crystallization is easier and easier, and its temperature and at the same, thermal glass stability parameter K H glass are lowering (Fig. 7). On the other hand, crystallization degree, measured by heat of DSC exothermic peak area, is increasing (Fig. 4). The crystallization peak becomes narrow and sharp (Fig. 4), which means the change of crystallization kinetics from slow to very fast, due to increasing mobility and easer rearrangement of elements in the structure, when concentration of sodium is increasing (Fig. 5).
Generally, results of the investigation of the thermal properties of Fe2O3–P2O5 glass did not confirm the earlier supposition on octahedral coordination of both Fe2+ and Fe3+ being the result of spectroscopic measurement interpretation [18, 19].
Phosphate glasses exhibit a nonlinearity of change the properties with their chemical composition change. The rapid change of the function of phosphate glass property versus composition is termed the phosphate anomaly, by analogy to the commonly-known boron or alumina anomaly connected with the change in their coordination number 4–6 and transition of the cation from network former to modifier position. The phosphate anomaly is an effect of the structure rebuilding, under the change of the proportions of components content, as for example, transition from ultra- to poly- to pyro- and/or ortho-phosphate condensation state accompanied by diminishing the number of the surrounding bridging oxides Q n , from Q 3 to Q 0. The investigation of the thermal properties of iron phosphate glasses proved that they also exhibit phosphate anomaly effect. This peculiarity explains the large capacity of incorporation of salt waste as Na2SO4 of the Fe2O3–P2O5 glasses.
Conclusions
Thermal properties of 37Fe2O3–63P2O5 (mol%) glass, with increasing content of Na2O added to it as Na2SO4, being the simulator of the radioactive salt waste, were investigated. It was demonstrated that the change of the properties with the glass chemical composition change exhibits a nonlinearity, being the effect of internal structure rebuilding, accompanying the sodium content increase.
The phosphate glasses exhibit effect of the structure rebuilding, under the components content proportion change, as transition from ultra- to poly- to pyro- and/or ortho-phosphate condensation state accompanied by diminishing the number of the surrounding bridging oxides Q n, from Q 3 to Q 0, termed phosphate anomaly. The investigation of the thermal properties of iron phosphate glasses proved that they also exhibit the phosphate anomaly effect accompanied by structure expansion and molar volume increase. This peculiarity explains the big capacity of the Fe2O3–P2O5 glasses for incorporation of salt waste as Na2SO4.
References
Donald W. Immobilisation of radioactive and non-radioactive wastes in glass-based systems: an overview. Glass Technol. 2007;48:155–63.
Ojovan MI, Lee WE. An introduction to nuclear waste immobilisation. Amsterdam: Elsevier Science; 2005.
Brow RK. Review: the structure of simple phosphate glasses. J Non-Cryst Solids. 2000;263, 264:1–28.
Kim CW, Day DE. Immobilization of Hanford LAW in iron phosphate glasses. J Non-Cryst Solids. 2003;331:20–31.
Bingham PA, Hand RJ, Scales CR. Immobilisation of simulated plutonium-contaminated material in phosphate glasses: an initial scoping study. Mater Res Soc Symp Proc. 2006;932:345–52.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Czech J Phys B. 1972;22:1187–96.
Cabral AA, Cardoso AAD, Zanotto ED. Glass-forming ability versus stability of silicate glasses I. Experimental test. J Non-Cryst Solids. 2003;320:1–8.
Lin SE, Cheng YR, Wei WCJ. Synthesis and long-term test of borosilicate-based sealing glass for solid oxide fuel cells. J Eur Ceram Soc. 2011;31:1975–85.
Nascimento MLF, Souza LA, Ferreira EB, Zanotto ED. Can glass stability parameters infer glass forming ability? J Non-Cryst Solids. 2005;351:3296–308.
Joseph K, Ghosh S, Govindan Kutty KV, Vasudeva Rao PR. Crystallization kinetics, stability and glass forming ability of iron phosphate and cesium loaded iron phosphate glasses. J Nucl Mater. 2012;426:223–39.
Biningham PA, Barney ER. Structure of iron phosphate glasses modified by alkali and alkaline earth additions: neutron and X-ray diffraction studies. J Phys. 2012;24:175403–16.
Stoch L, Wacławska I, Środa M. Thermal study of the influence of chemical bond iconicity on the glass transformation in (Na2O, CaO, MgO)–Al2O3–SiO2 glasses. J Therm Anal Calorim. 2004;77:57–63.
Stoch L. Thermochemistry of solids with flexible structures. J Therm Anal Calorim. 1998;54:9–24.
Stoch L. Thermal analysis and thermochemistry of vitreous to crystalline state transition. J Therm Anal Calorim. 2004;77:7–16.
Stoch L, Sroda M, Olejniczak Z. Synthesis and structure of AlPO4–BPO4–SiO2 glasses, ceramics, Polish Ceramic Bulletin, Polish Academy of Science, Krakow. 1997;15:119–128.
Brow RK. The nature of alumina in phosphate glass I. Properties of sodium aluminophosphate glass. J Am Ceram Soc. 1993;76:913–8.
Jin Y, Jiang D, Chen X, Bian B, Huawy X. Raman spectrum studies of the glasses in the system Na2O–Al2O3–P2O5. J Non-Cryst Solids. 1986;80:147–51.
Karabulut M, et al. An investigation of the local iron environment in iron phosphate glasses having different Fe(II) concentrations. J Non-Cryst Solids. 2002;306:182–92.
Hoppe U, Karabut M, Metwalli E, Brow RK, Jovari P. The Fe–O coordination in phosphate glasses by X-ray diffraction with high energy photons. J Phys. 2003;15:6143–53.
Stoch L, Stoch P. Crystal structures formation in glass from view of HRTEM. J Therm Anal Calorim. 2007;88:577–82.
Stoch L, Stoch P. Significance of crystallochemical factors in chemical reactions into the structure of solids. J Therm Anal Calorim. 2012;109:763–6.
Hannant OM, Bingham PA, Hand RJ, Forder SD. The structural properties of iron in vitrified waste ashes, glass technology. J Glass Sci Technol. 2008;49:27–32.
Acknowledgements
The work was supported partially by the National Science Centre of Poland Project No. N N507 235740 and partially by the National Centre for Research and Development of Poland as a part of strategic research project Technologies Supporting Development of Safe Nuclear Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Stoch, P., Ciecinska, M. & Stoch, A. Thermal properties of phosphate glasses for salt waste immobilization. J Therm Anal Calorim 117, 197–204 (2014). https://doi.org/10.1007/s10973-014-3698-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3698-2