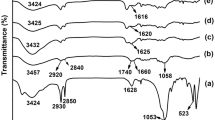
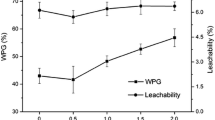
Abstract
Melamine formaldehyde-furfuryl alcohol copolymer was impregnated into softwood in combination with 1,3-dimethylol-4,5-dihydroxy ethyleneurea, a crosslinking agent, nanoclay, and a renewable polymer, collected as gum from a local plant (Moringa oleifera) under vacuum condition and polymerized by catalyst heat treatment. Fourier-transform infrared spectroscopy, X-ray diffractometry, and scanning electron microscopy were used to characterize the nanocomposites. Transmission electron microscopy showed uniform distribution of nanoclay in the composites. The mechanical properties were improved after the addition of plant polymer. The plant polymer had a marked influence on the flammability and thermal stability of the prepared composites. The apparent activation energy was determined by Ozawa-Flynn-Wall’s and Vyazovkin methods. The activation energy of the composites decreased up to a certain decomposed fraction thereafter it remained constant. Higher the plant polymer content higher was the activation energy of the prepared composites which indicated a better interfacial adhesion and thermal stability.













Similar content being viewed by others
References
Devi RR, Ali I, Maji TK. Chemical modification of rubber wood with styrene in combination with a crosslinker: effect on dimensional stability and strength property. Bioresour Technol. 2003;88:185–8.
Kim JW, Carlborn K, Matuana LM, Heiden PA. Thermoplastic modification of urea–formaldehyde wood adhesives to improve moisture resistance. J Appl Polym Sci. 2006;101:4222–9.
Baysal E, Kiyoka S, Mustafa O, Yalinkilic K. Dimensional stabilization of wood treated with furfuryl alcohol catalysed by borates. Wood Sci Technol. 2004;38:405–15.
Mantanis GI, Young RA, Rowell RM. Swelling of wood: part I Swelling in water. Wood Sci Technol. 1994;28:119–34.
Mantanis GI, Young RA, Rowell RM. Swelling of wood: part II Swelling in organic liquids. Holzforschung. 1994;48:480–90.
Lande S, Westin M, Schneider M. Properties of Furfurylated Wood. Scand J For Res. 2004;19:22–30.
Schneider MH. New cell wall and cell lumen wood polymer composites. Wood Sci Technol. 1995;29:135–58.
Esteves B, Nunes L, Pereira H. Properties of furfurylated wood (Pinus pinaster). Eur J Wood Prod. 2011;69:521–5.
Gindl W, Zargar-Yaghubi F, Wimmer R. Impregnation of softwood cell walls with melamine-formaldehyde resin. Bioresour Technol. 2003;87:325–30.
Watanabe M, Sakurai M, Maeda M. Preparation of ammonium polyphosphate and its application to Flame retardant. Phosphorus Res Bull. 2009;23:35–44.
Baysal E. Determination of oxygen index levels and thermal analysis of scots pine (pinus sylvestris l.) impregnated with melamine formaldehyde-boron combinations. J Fire Sci. 2002;20:373–89.
Jana T, Roy BC, Maiti S. Biodegradable film Modification of the biodegradable film for fire retardancy. Polym Degrad Stab. 2000;69:79–82.
Ghosh SN, Maiti S. Adhesive performance, flammability Evaluation and biodegradation study of Plant polymer blends. Eur Polym J. 1998;34:849–54.
Arora S, Kumar M, Kumar M. Catalytic effect of bases in impregnation of guanidine nitrate on Poplar (Populus) wood Flammability and multiple heating rate kinetic study. J Therm Anal Calorim. 2012;107:1277–86.
Ak M, Cilgi GK, Kuru FD, Cetisli H. Thermal decomposition kinetics of polypyrrole and its star shaped copolymer. J Therm Anal Calorim. 2013;111:1627–32.
Zhou Q, Xanthos M. Nanosize and microsize clay effects on the kinetics of the thermal degradation of polylactides. Polym Degrad Stab. 2009;94:327–38.
Zhao L, Cao Z, Fang Z, Guo Z. Influence of fullerene on the kinetics of thermal and thermo-oxidative degradation of high-density polyethylene by capturing free radicals. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-3158-4.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solidstate reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Vyazovkin S, Sbirrazzuoli N. Estimating the activation energy for Non-isothermal crystallization of Polymer melts. J Therm Anal Calorim. 2003;72:681–6.
Hazarika A, Maji TK. Effect of different crosslinkers on properties of melamine formaldehyde-furfuryl alcohol copolymer/montmorillonite impregnated softwood (ficus hispida). Polym Eng Sci. 2012;. doi:10.1002/pen.23391.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A. 1966;70A:487–523.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Xie Y, Xiao Z, Grüneberg T, Militz H, Hill CAS, Steuernagel L, Mai C. Effects of chemical modification of wood particles with glutaraldehyde and 1,3-dimethylol-4,5-dihydroxyethyleneurea on properties of the resulting polypropylene composites. Compos Sci Technol. 2010;70:2003–11.
Devi RR, Maji TK. Chemical modification of simul wood with styrene–acrylonitrile copolymer and organically modified nanoclay. Wood Sci Technol. 2012;46:299–315.
Lori JA, Myina OM, Ekanem EJ, Lawal AO. Structural and adsorption characteristics of carbon adsorbent synthesized from polyfurfuryl alcohol with kaolinite template. Res J Appl Sci Eng Technol. 2011;3:440–6.
Jang TR, Sheu TC, Sheu JJ, Chen CC. Crosslinking of Cotton Fabrics Premercerized with Different Alkalis, Part III: crosslinking and Physical Properties of DMDHEU-Treated Fabrics. Text Res J. 1993;63:679–86.
Deka BK, Maji TK. Effect of coupling agent and nanoclay on properties of HDPE, LDPE, PP, PVC blend and Phargamites karka nanocomposite. Compos Sci Technol. 2010;70:1755–61.
Lu WH, Zhao GJ, Xue ZH. Preparation and characterization of wood/montmorillonite Nanocomposites. For Stud China. 2006;8:35–40.
Devi RR, Maji TK. Preparation and characterization of wood/styrene-acrylonitrile co-polymer/MMT nanocomposite. J Appl Polym Sci. 2011;122:2099–109.
Cai X, Riedl B, Zhang SY, Wan H. The impact of the nature of nanofillers on the performance of wood polymer nanocomposites. Compos Part A. 2008;39:727–37.
Gilman JW, Jackson CL, Morgan AB, Harris RH, Manias E, Giannelis EP, Wuthenow M, Hilton D, Phillips S. Flammability properties of polymer-layered-silicate nanocomposites: polypropylene and polystyrene nanocomposites. Chem Mater. 2000;12:1866–73.
Qin H, Zhang S, Zhao C, Feng M, Yang M, Shu Z. Thermal stability and flammability of polypropylene/montmorillonite composites. Polym Degrad Stab. 2004;85:807–13.
Fung KL, Li RKY, Tjong SC. Interface modification on the properties of sisal fiber-reinforced polypropylene composites. J Appl Polym Sci. 2002;85:169–76.
Doh GH, Lee SY, Kang IA, Kong YT. Thermal behavior of liquefied wood polymer composites (LWPC). Compos Struct. 2005;68:103–8.
Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stab. 2008;93:90–8.
Kim HS, Yang HS, Kim HJ, Park HJ. Thermogravimetric analysis of rice husk Flour filled thermoplastic polymer Composites. J Therm Anal Calorim. 2004;76:395–404.
Hsiue GH, Wei HF, Shiao SJ, Kuo WJ, Sha YA. Chemical modification of dicyclopentadiene-based epoxy resins to improve compatibility and thermal properties. Polym Degrad Stab. 2001;73:309–18.
Vyazovkin S, Sbirrazzuoli N. Isoconversional method to explore the mechanism and kinetics of multi-step epoxy cures. Macromol Rapid Commun. 1999;20:387–9.
Acknowledgements
University grant commission (UGC) is acknowledged for financial support in the form of institutional fellowship to one of the authors (AH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazarika, A., Maji, T.K. Thermal decomposition kinetics, flammability, and mechanical property study of wood polymer nanocomposite. J Therm Anal Calorim 115, 1679–1691 (2014). https://doi.org/10.1007/s10973-013-3394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3394-7