Abstract
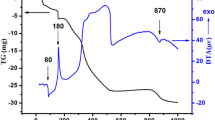
Perovskite-type strontium stannate, SrSnO3, is of technological interest because of its potential applications. In the present work, Fe(III)-doped SrSnO3 perovskites were synthesized using Sn metal and the polymeric precursor method, derived from the Pechini method. Thermogravimetric evaluations in N2 and O2 atmospheres were performed to evaluate the possible formation of oxygen vacancies resulting from Fe(III) doping. The X-ray diffraction patterns showed the formation of an orthorhombic structure with high crystallinity after calcination at 1,073 K. The first thermal analysis, in a N2 atmosphere, led to the partial elimination of strontium carbonate and hydroxyl groups, while the thermal analysis in an O2 atmosphere led to an increase in the mass, indicating O2 adsorption on the lattice, which could be related to the presence of Fe(III) and Fe(IV) in the perovskite structure. Moreover, the thermal analysis changed the symmetry of the iron-doped perovskites.





Similar content being viewed by others
References
Peña MA, Fierro JLG. Chemical structures and performance of perovskite oxides. Chem Rev. 2001;101:1981–2017.
Kakihana M, Yoshimura M. Synthesis and characteristic of complex multicomponent oxides prepared by polymer complex method. Bull Chem Soc Jpn. 1999;72:1427–43.
Alves MCF, Nascimento MR, Lima SJ, Pizani PS, Espinosa JWM, Longo E, Soledade LEB, Souza AG, Santos IMG. Influence of synthesis conditions on carbonate entrapment in perovskite SrSnO3. Mater Lett. 2009;63:118–20.
Oliveira ALM, Silva MRS, Sales H, Longo E, Maia AS, Souza AG, Santos IMG. Effect of the composition on the thermal behavior of the SrSn1−x Ti x O3 precursor prepared by the polymeric precursor method. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3051-1.
Lee CW, Kim DW, Cho IS, Park S, Shin SS, Seo SW, Hong KS. Simple synthesis and characterization of SrSnO3 nanoparticles with enhanced photocatalytic activity. Int J Hydrogen Energy. 2013;37(14):10557–63.
Liu HR, Yang JH, Xiang HJ, Gong XG, Wei SH. Origin of the superior conductivity of perovskite Ba(Sr)SnO3. Appl Phys Lett. 2013. doi:10.1063/1.4798325.
Prathiba G, Venkatesh S, Kumar NH. The optical and electronic transport properties of SrSn0.95Fe0.05O3 films. Solid State Phys. 2012;1447:1219–20.
Wang SF, Hsu YF, Yeh CT, Huang CC, Lu HC. Characteristics of SrCo1−x Sn x O3−delta cathode materials for use in solid oxide fuel cells. Solid State Ion. 2012;227:10–6.
Ouni S, Nouri S, Rohlicek J, Ben HR. Structural and electrical properties of the sol–gel prepared Sr1−x Er x SnO3−delta compounds. J Solid State Chem. 2012;192:132–8.
Patel DK, Rajeswari B, Sudarsan V, Vatsa RK, Kadam RM, Kulshreshtha SK. Structural, luminescence and EPR studies on SrSnO3 nanorods doped with europium ions. J Chem Soc Dalton. 2012;41(39):12023–30.
Qinzhuang L, Jianming D, Xiaobo Z, Guangping Z, Zhongliang L, Guohua D. Perovskite-type transparent and conductive oxide films: Sb- and Nd-doped SrSnO3. Thin Solid Films. 2011;519:6059–63.
Lim CS. A facile solvothermal process to synthesize MSnO3 (M = Sr, Mg) nanoparticles assisted by microwave irradiation. Asian J Chem. 2013;25(6):3211–4.
Ahmed J, Blakely CK, Bruno SR, Poltavets VV. Synthesis of MSnO3 (M = Ba, Sr) nanoparticles by reverse micelle method and particle size distribution analysis by whole powder pattern modeling. Mater Res Bull. 2012;47(9):2282–7.
Alves MCF, Marinho RMM, Casali GP, Siu-Li M, Deputier S, Guilloux-Viry M, Souza AG, Longo E, Weber IT, Santos IMG. Influence of the network modifier on the characteristics of MSnO3 (M = Sr and, Ca) thin films synthesized by chemical solution deposition. J Solid State Chem. 2013;199:34–41.
Gellings PJ, Bouwmeester HJM. Solid state aspects of oxidation catalysis. Catal Today. 2000;58:1–53.
Longo VM, Cavalcante LS, Erlo R, Mastelaro VR, Figueiredo AT, Sambrano JR, Lázaro S, Fretias AZ, Gomes L, Vieira ND Jr, Valera JA, Longo E. Strong violet–blue light photoluminescence emission at room temperature in SrZrO3: joint experimental and theoretical study. Acta Mater. 2008;56:2191–202.
Mueller DN, Souza RA, Yoo H, Martin M. Phase stability and oxygen nonstoichiometry of highly oxygen-deficient perovskite-type oxides: a case study of (Ba,Sr)(Co,Fe)O3−δ. Chem Mater. 2012;24:269–74.
Arias AG, Torres C, Francisco C, Muñoz JM, Gómez PH, Alejos O, Montero O, Iñiguez JI. Defect concentration in Ti-substituted YIG from TG curves. J Therm Anal Calorim. 2006;86(1):195–8.
Vieira FTG, Oliveira ALM, Melo DS, Lima SJG, Longo E, Maia AS, Souza AG, Santos IMG. Crystallization study of SrSnO3:Fe. J Therm Anal Calorim. 2011;106:507–12.
Misra S, Gnanasekar KI, Rao RVS, Jayaraman V, Gnanasekaran T. Electrical conductivity and oxygen sensing behavior of SrSn1−x Fe x O3-delta (x = 0–0.2). J Alloy Compd. 2010;506(1):285–92.
Prathiba G, Venkatesh S, Kumar NH. Structural, magnetic and semiconducting proprieties of Fe doped SrSnO3. Solid State Commun. 2010;150:1436–8.
Thangadurai V, Huggins RA, Weppner W. Use of simple ac technique to determine the ionic and electronic conductivities in pure and Fe-substituted SrSnO3 perovskites. J Power Sources. 2002;108:64–9.
Thangadurai V, Beurmam PS, Weppner W. Mixed oxide ion and electronic conductivity in perovskite-type SrSnO3 by Fe substitution. Mater Sci Eng B. 2003;100:18–22.
Beurmam PS, Thangadurai V, Weppner W. Phase transitions in the SrSnO3–SrFeO3 solid solutions: X-ray diffraction and Mossbauer studies. J Solid State Chem. 2003;174:392–402.
Roh KS, Ryu KH, Yo CH. Nonstoichiometric and physical properties of the SrSn1−x Fe x O3−x system. J Solid State Chem. 1999;142:288–93.
Pechini MP. Method of preparing lead and alkaline: earth titanates and niobates and coating method using the same to from a capacitor. 1967. US Patent no. 3.330.697.
Alves MCF, Souza SC, Silva MRS, Paris EC, Lima SJG, Gomes RM, Longo E, Souza AG, Santos IMG. Thermal analysis applied in the crystallization study of SrSnO3. J Therm Anal Calorim. 2009;97:179–83.
Kim MG, Cho HS, Yo CH. Fe K-edg X-ray absorption (XANES/EXAFS) spectroscopic study of the nonstoichiometric SrFe1−x Sn x O3−x system. J Phys Chem Solids. 1998;59:1369–81.
Kröger FA, Vink HJ. Relations between the concentrations of imperfections in crystalline solids. Solid State Phys. 1956;5(3):208–23.
Melo D, Marinho RMM, Vieira FTG, Lima SJG, Longo E, Souza AG, Maia AS, Santos IMG. Influence of Cu(II) in the SrSnO3 crystallization. J Therm Anal Calorim. 2011;106:513–7.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. New York: Wiley; 1997.
Moreira E, Henriques JM, Azevedo DL, Caetano EWS, Freire VN, Albuquerque EL. Structural, optoelectronic, infrared and Raman spectra of orthorhombic SrSnO3 from DFT calculations. J Solid State Chem. 2011;184:921–8.
Ranson P, Ouillon R, Pinan-Lucarre JP, Pruzan P, Mishra SK, Ranjan R, Pandey D. The phases of the system Sr1−x Ca x TiO3: a Raman scattering study. J Raman Spectrosc. 2005;36:898–911.
Lin CC, Liu L. Post-aragonite phase transitions in strontianite and cerussite: a high-pressure Raman spectroscopic study. J Phys Chem Solids. 1997;58:977–87.
Alves MCF, Souza SC, Lima HHS, Nascimento MR, Silva MRS, Espinola JWM, Lima SJG, Longo E, Pizani PS, Soledade LEB, Souza AG, Santos IMG. Influence of the modifier on the short and long range disorder of stannate perovskites. J Alloy Compd. 2009;476:507–12.
Tarrida M, Larguem H, Madon M. Structural investigations of (Ca, Sr)ZrO3 and Ca(Sn, Zr)O3 perovskite compounds. Phys Chem Miner. 2009;36:403–13.
Siny IG, Katiyar RS, Bhalla AS. Cation arrangement in the complex perovskites and vibrational spectra. J Raman Spectrosc. 1998;29:385–90.
Siny IG, Tao W, Katiyar RS, Guo R, Bhalla AS. Raman spectroscopy of Mg–Ta order-disorder in BaMg1/3Ta2/3O3. J Phys Chem Solids. 1998;59(2):181–95.
Nilsen WG, Skinner JG. Raman spectrum of strontium titanate. J Chem Phys. 1968;48(5):2240–8.
Glerup M, Knight KS, Poulsen FW. High temperature structural phase transitions in SrSnO3. Mater Res Bull. 2005;40:507–20.
Mitchell RH. Perovskites: modern and ancient. Winnipeg: Kromar Printing Ltd; 2002.
Acknowledgements
The authors acknowledge RECAM/CNPq/MCT, INCT/CNPq/MCT, and PROINFRA/FINEP/MCT for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucena, G.L., Maia, A.S., Souza, A.G. et al. Structural changes in Fe-doped SrSnO3 perovskites during thermal analysis. J Therm Anal Calorim 115, 137–144 (2014). https://doi.org/10.1007/s10973-013-3313-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3313-y