Abstract
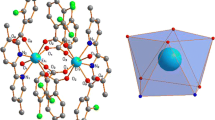
Solid-state compounds of yttrium and lanthanide chelates of ethylenediaminetetraacetic acid have been synthesized. Simultaneous thermogravimetry and differential scanning calorimetry (TG-DSC), theoretical and experimental infrared spectroscopy (FTIR), elemental analysis, complexometry and TG-DSC coupled to FTIR were used to characterize and to study the thermal decomposition of these compounds. The results provided information about the composition, dehydration, thermal stability, thermal decomposition and identification of gaseous products evolved during the thermal decomposition of these compounds. The theoretical and experimental spectroscopic data suggest the possible modes of coordination of the ligand with the lanthanum and terbium metal ions.




Similar content being viewed by others
References
Moeller T, Moss FAJ, Marshall RH. Observations on the rare earths. LXVI. Some characteristics of ethylenediaminetetraacetic acid chelates of certain rare earth metal ions. J Am Chem Soc. 1955;77:3182–6.
Kolat RS, Powell JE. The solid rare earth chelates of ethylenediaminetetraacetic acid. Inorg Chem. 1962;1:485–90.
Charles RG. Thermal properties of some solid neodymium complexes derived from ethylenediaminetetraacetic acid. J Inorg Nucl Chem. 1966;28:407–14.
Bhat TR, Krishna Iyer R. Studies on EDTA complexes–VIII Thermal behaviour in air and nitrogen atmosphere of some metal-EDTA complexes. J Inorg Nucl Chem. 1967;29:179–85.
Wendlandt WW. Thermogravimetric and differential thermal analysis of (ethylenedinitrilo)tetraacetic acid. Anal Chem. 1960;32:848–50.
Wendlandt WW, Horton GR. Differential thermal analysis of some transition metal ethylenediaminetetraacetic acid chelates. Nature. 1960;187:769–70.
Morris ML, Dunhan RW, Wendlandt WW. The thermal properties of some pentadentate and hexadentate cobalt complexes of ethylenediaminetetraacetic acid and hydroxyethylenediaminetetraacetic acid. J Inorg Nucl Chem. 1961;20:274–82.
Mercadante A, Ionashiro M, de Oliveira LCS, Ribeiro CA, Moscardini D′Assunção L. Preparation and thermal decomposition of solid-state lanthanide (III) and yttrium (III) chelates of ethylenediaminetetraacetic acid. Thermochim Acta. 1993;216:267–77.
Vikram L, Raju B, Ragul R, Sivasankar BN. Thermal degradation studies on some lanthanide-EDTA complexes containing hydrazinium cation. J Therm Anal Cal. 2008;93:987–91.
Kumar AS, Indrasenan P. Thermal decomposition studies of lanthanide (III) complexes of EDTA. Asian J Chem. 2008;20:5178–86.
Flaschka HA. EDTA titrations: an introduction to theory and practice. 2nd ed. Oxford: Pergamon Press; 1964.
Ionashiro M, Graner CAF, Zuanon Netto J. Titulação complexométrica de lantanídeos e ítrio. Ecl Quim. 1983;8:29–32.
Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–52.
Lee C, Yang W, Parr RG. Development of the colle-salvetti correlation energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–9.
Treu-Filho O, Pinheiro JC, da Costa EB, Ferreira JEV, de Figueiredo AF, Kondo RT, de Lucca Neto VA, de Souza RA, Legendre AO, Mauro AE. Experimental and theoretical study of the compound [Pd(dmba)(NCO)(imz)]. J Mol Struct. 2007;829:195–201.
Gigante AC, Gomes DJC, Lima LS, Caires FJ, Treu-Filho O, Ionashiro M. Synthesis, thermal properties and spectroscopic study of solid mandelate of light trivalent lanthanides. Thermochim Acta. 2012;536:6–14.
Treu-Filho O, Pinheiro JC, Kondo RT, Marques RFC, Paiva-Santos CO, Davolos MR, Jafelicci M Jr. Gaussian basis sets to the theoretical study of the electronic structure of perovskite (LaMnO3). J Mol Struc (Theochem). 2003;631:93–9.
Treu-Filho O, Pinheiro JC, Kondo RT. Designing Gaussian basis sets to the theoretical study of the piezoelectric effect of perovskite (BaTiO3). J Mol Struct. 2004;671:71–5.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Revision A. 02. Wallingford, CT: Gaussian Inc.; 2009.
Goodson DZ, Sarpal SK, Wolfsberg M. Influence on isotope effect calculations of the method of obtaining force constants from vibrational data. J Phys Chem. 1982;86:659–63.
Schelegel HB. In: Bertran J, editor. New theoretical concepts for understanding organic reactions. The Netherlands: Academic; 1989. p. 33–53.
Dennington R, Keith T, Millam J. GaussView. Version 5. 0. 8. Shawnee Mission, KS: Semichem Inc.; 2000–2008.
Acknowledgements
The authors thank FAPESP, CNPq and CAPES foundations (Brazil) for financial support. This research was supported by resources supplied by the Center for Scientific Computing (NCC/GridUNESP) of the São Paulo State University (UNESP), Instituto de Química de Araraquara, UNESP—Campus de Araraquara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gigante, A.C., Caires, F.J., Gomes, D.J.C. et al. Spectroscopic study and thermal behavior of trivalent lanthanides and yttrium(III) chelates of EDTA using TG-DSC, FTIR, and TG-DSC coupled to FTIR. J Therm Anal Calorim 115, 127–135 (2014). https://doi.org/10.1007/s10973-013-3284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3284-z