Abstract
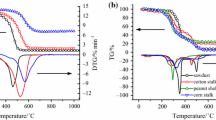
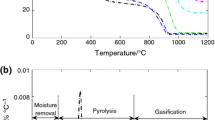
The growing attention on carbon dioxide emissions by coal-fired power plants has led to an increase in coal blending with biomass. Coal blending with biomass is practiced for economic considerations keeping coal quality in mind. Thus, the thermal parameters involved during pyrolysis and combustion of coal, miscanthus, and tobacco stems as measured in thermogravimetric (TG) analysis experiments can be applied. There is a need to determine the relationships that exist between the measured values of TG thermal parameters in individual coal and biomass and those in the blends. The TG thermal parameters are mass loss, T i, T f, T p, t f, and the maximum mass loss rate (DTGmax). In this study, a series of lignite coal and biomass blends with and without mineral content were investigated. Thermal parameters for the blends were measured to examine the additive and nonadditive nature of results obtained under both pyrolysis and combustion conditions using TG analysis. The nonadditive nature of coal blended samples may be due to the mixed catalysts effect (synergism). Thus, the mixed catalysts play an important nonadditive role in several reaction pathways.





Similar content being viewed by others
References
Bradbury AGW, Sakai Y, Shafizadeh F. A kinetic model for pyrolysis of cellulose. J Appl Polym Sci. 1979;23:3271–80.
Hsisheng T, Chou Wei Y. Thermogravimetric studies on the kinetics of rice hull pyrolysis and the influence of water treatment. Ind Eng Chem Res. 1998;37:3806–11.
Raveendran K, Ganesh A, Khilar KC. Influence of mineral matter on biomass pyrolysis characteristics. Fuel. 1995;74(12):1812.
Fahmi R, Bridgwater AV, Darvell LI, Jones JM, Yates N, Thain S, Donnison IS. The effect of alkali metals on combustion and pyrolysis of Lolium an Festuca grasses, switchgrass and willow. Fuel. 2007;86:1560–9.
Raveendran K, Ganesh A, Khilar KC. Influence of mineral matter on biomass pyrolysis characteristics. Fuel. 1995;74(12):1812.
Czernik S, Bridgwater AV. Overview of application of biomass fast pyrolysis oil. Energy Fuels. 2004;18:590.
Liden AG, Berruti F, Scott D. A kinetics model for the production of liquids from the flash pyrolysis of biomass. Chem Eng Commun. 1998;65:207–21.
Hague RA. The pre-treatment and pyrolysing of biomass for the production of liquids for fuel and speciality chemicals. Ph.D. thesis, Aston University; 1998. p. 35–90.
Barrena R, Vazquez F, Sanchez A. Dehydrogenase activity as a method for monitoring the composting process. Bioresour Technol. 2008;99(4):905–8.
Pan Wei-Ping, Gan Yaodong, Serageldin Mohamad A. A study of thermal analytical values for coal blends in air atmosphere. Thermochemica Acta. 1991;180:203–17.
Demirbaş Ayhan. Sustainable cofiring of biomass with coal [J]. Energy Convers Manage. 2003;44:1465–79.
Chen Yong, Mori Shigeikatsu, Pan Wei-Ping. Estimating the combustibility of various coals by TG-DTA. Energy Fuels. 1995;9:71–4.
Orfao JJM, Antunes FJA, Figueiredo JL. Pyrolysis kinetics of lignocellulosic materials—three independent reactions model. Fuel. 1999;78:349–58.
Fisher T, Hajaligol M, Waymack B, Kellog D. Pyrolysis behavior and kinetics of biomass derived materials. J Anal Appl Pyrol. 2002;62:331–49.
Tsamba AJ, Yang WH, Blasiak W. Pyrolysis characteristics and global kinetics of coconut and cashew nut shells. Fuel Process Technol. 2006;87:523–30.
Gherri Paolo, Ricca Leandro, Angelini Luciana. Themal analysis of biomass and corresponding pyrolysis products. Fuel. 1996;75(5):565–73.
Sung YJ, Seo YB. Thermogravimetric study on stem biomass of Nicotiana tabacum. Thermochimica Acta. 2009;486:1–4.
Jian-yuan Cheng, Xue-xing Sun. Determination of the devolatilization index and combustion characteristic index of pulverized coals. Power Eng. 1987;7(5):33–6.
Li X, Ma B, Li X, Hu Z, Wang X. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta. 2006;441:79–83.
Youssef MA, Wahid SS, Mohamed MA, et al. Experimental study on Egyptian biomass combustion in circulating fluidized bed. Appl Energy. 2009;86:2644–50.
Acknowledgments
This research was partially supported by the 111 Project (B12034) and National Natural Science Foundation of China (No. 51076043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, Y., Zhong, L. et al. Synergistic effects of mineral matter on the combustion of coal blended with biomass. J Therm Anal Calorim 113, 489–496 (2013). https://doi.org/10.1007/s10973-013-3162-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3162-8