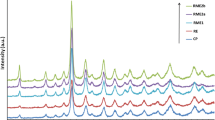
Abstract
In this study, two compounds presenting characteristics different from each other were produced from there action between hydrated Eu3+ sulfate and Ba2+ diphenylamine-4-sulfonate using, respectively, aqueous solution for producing the Eu(C12H10NSO3)3·7H2O (A) compound and water/ethyl alcohol (7:1) solution for the Eu(C12H10NSO3)3·5H2O (B) production. The presence of alcohol molecules in the solution will interfere in the structural arrangement of anionic surfactant DAS− (diphenylamine-4-sulfonate) around the metal ions Eu3+ allowing differentiation in the stoichiometric formulas, morphology, and thermal properties of these compounds and their derivatives. Thus, when treating both compounds under oxidizing atmosphere, we found different temperatures of the water loss and conversion of the intermediate pair oxydisulfate [Eu2O(SO4)2]/dioxysulfate [(Eu2O2SO4)]. However, the effect of water/surfactant/alcohol interactions in the metal ion structural arrangement becomes still more evident under reducing atmosphere. After this thermal treatment, significant changes were observed in the morphological characteristics and physical properties of the (Eu2O2S oxysulfide) in compound B with respect to compound A.













Similar content being viewed by others
References
Zhang H, Jiang H, Gong H, Sun Z-L. Characteristics of thermal decomposition products of rare earth, alkali earth metal and transition metal p-toluenesulfonates. J Therm Anal Calorim. 2005;79:731–5. doi:10.1007/s10973-005-0604-y.
Wang M, Jiang H, Wang Z-C. Dehydration studies of Co(II), Cu(II) and Zn(II) methanesulfonates. J Therm Anal Calorim. 2006;8:751–4. doi:10.1007/s10973-005-7064-2.
Delgado S, Molina-Ontoria A, Medina M-E, Pastor C-J, Jimenez-Aparicio R, Priego JL. An unexpected sulfinate–sulfonate mixed coordination polymer of copper(II). Inorg Chem Commun. 2006;9:1289–92. doi:10.1016/j.inoche.2006.06.026.
Yang J, Li L, Ma J-F, Liu Y-Y, Ma J-C. Two new barium sulfonates with pillared layered structures. J Mol Struct. 2006;788:43–8. doi:10.1016/j.molstruc.2005.11.016.
Selvan R-K, Gedanken A. Synthesis and characterization of hierarchically structured La2O2M@C:Eu3+. Eur J Inorg Chem. 2010;0: 5685–91. doi: 10.1002/ejic.201000632.
Llanos J, Sánchez V, Mujica C, Buljan A. Synthesis, physical properties, and electronic structure of rare earths oxysulfides Ln2O2S (Ln=Sm, Eu). Mater Res Bull. 2002;379:2285–91. doi:10.1016/S0025-5408(02)00936-4.
Bang J, Abboudi M, Abrams B, Hooloway P. Combustion synthesis of Eu-, Tb- and Tm- doped Ln2O2S (Ln=Y, La, Gd) phosphors. J Lumin. 2004;106:177–85. doi:10.1016/j.jlumin.2003.09.005.
Zhao F, Yuan M, Zhang W, Gao S. Monodisperse lanthanide oxysulfide nanocrystals. J Am Chem Soc. 2006;128:11758–9. doi:10.1021/ja0638410.
Ikeue K, Kawano T, Eto M, Zhang D, Machida M. X-ray structural study on the different redox behaviors of La and Pr oxysulfates/oxysulfides. J Alloys Compd. 2008;451:338–40. doi:10.1016/j.jallcom.2007.04.145.
Zhang D, Yoshioka F, Ikeue K, Machida M. Synthesis and oxygen release/storage properties of Ce-substituted La-oxysulfates, (La1−X Ce X )O2SO4. Chem Mater. 2008;20:6697–703. doi:10.1021/cm801629b.
Machida M, Kawamura K, Ito K, Ikeue K. Large capacity oxygen storage by lanthanide oxysulfate/oxysulfide systems. Chem Mater. 2005;17:1487–92. doi:10.1021/cm0479640.
Machida M, Kawano T, Eto M, Zhang D, Ikeue K. Ln dependence of the large-capacity oxygen storage/release property of Ln oxysulfate/oxysulfide systems. Chem Mater. 2007;19:954–60. doi:10.1021/cm062625n.
Machado L-C, Marins A-A-L, Muri E-J-B, Biondo A, Matos J-R, Mazali I-O. Complexation of the Fe(III) and Fe(II) sulphates with diphenyl-4-amine barium sulphonate (DAS): synthesis, thermogravimetric and spectroscopic studies. J Therm Anal Calorim. 2009;97:289–96. doi:10.1007/s10973-009-0259-1.
Yang J, Ma J-F, Wu D-M, Guto L-P, Liu J-F. Syntheses, crystal structures and characterization of divalent transition metal sulfonate complexes with o-phenanthroline. J Mol Struct. 2003;657:333–41. doi:10.1016/S0022-2860(03)00428-9.
Charbonner F. Thermal behavior of some compounds of methanesulfonic acid with transition metals. Thermochim Acta. 1979;33:31–9. doi:10.1016/0040-6031(79)87027-6.
de Maria M-F-V, Matos J-R, de Farias R-F. Synthesis, characterization and a thermal (TG-DSC) study of gadolinium and lutetium methanesulfonate coordination compounds with pyridine-N-oxide and 2-, 3- and 4- picoline-N-oxides. J Serbian Chem Soc. 2005;70:1041–8. doi:10.2298/JSC0509041d.
de Moura M-F-V, Matos J-R, de Farias R-F. Thermal degradation study of gadolinium and lutetium methanesulfonates. Thermochim Acta. 2004;414:159–66. doi:10.1016/j.tca.2003.12.020.
Cao S-W, Zhu Y-J, Chang J. Fe3O4 polyhedral nanoparticles with a high magnetization synthesized in a mixed solvent ethylene glycol–water system. New J Chem. 2008;32:1526–30. doi:10.1039/b719436f.
Haines D-A, Chisholm J-A, Jones W, Motherwell W-D-S. Supramolecular synthon competition in organic sulfonates: a CSD survey. Cryst Eng Comm. 2004;6:584–8. doi:10.1039/b413797c.
dos Santos A-V, Matos J-R. Dehydration studies of rare earth p-toluenesulfonate hydrates by TG/DTG and DSC. J Alloys Compd. 2002;344:195–8. doi:10.1016/S0925-8388(02)00339-0.
dos Santos A-V. p-Toluene sulfonates of earth rare hydrated: synthesis, characterization and study thermoanalytic in different atmospheres. Ph.D. Thesis, Chemistry Institute of São Paulo, 1998.
Karsruhe FIZ, ICSD Collection code 95819.
Nakanishi K, Solomon P-H. Infrared absorption spectroscopy. San Francisco: Holden-Day; 1977.
Bellamy L-J. The infrared spectra of complex molecules. London: Chapman and Hall; 1980.
Braterman P-S, Xu Z-P. High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J Mater Chem. 2003;13:268–73. doi:10.1039/b207540g.
Beentjes P-C-J, Van DerBrand J, De Wiit J-H-W. Interaction of ester and acid groups containing organic compounds with iron oxide surfaces. J Adhes Sci Technol. 2006;20:1–18. doi:10.1163/156856106775212396.
Zhang H, Wen X, Wang Y. Synthesis and characterization of sulfate and dodecylbenzene sulfonate intercalated zinc-iron layered double hydroxides by one-step coprecipitation. J Solid State Chem. 2007;180:1636–47. doi:10.1016/j.jssc.2007.03.016.
JCPDS—International Centre for Diffraction Data, 1996;26:1418.
Suponitsky Y-L, Laptev V-I. Thermodynamics of the formation of oxosulfides of rare-earth elements. Russ Chem Bull. 1997;46:279–83. doi:1066-5285/97/4602-0279.
Leskelä M, Niinistö L. Thermal decomposition of europium sulfite trihydrate in carbon monoxide. Thermochim Acta. 1980;37:125–30. doi:10.1016/0040-6031(80)80032-3.
JCPDS—International Centre for Diffraction Data, 1996;34:392.
Machado L-C, D’azeredo M-T-O, Corrêa H-P-S, Matos J-R, Mazali I-O. Formation of oxysulfide Ln2O2S and oxysulfate Ln2O2SO4 phases in the thermal decomposition process of lanthanide sulfonates (Ln=La, Sm). J Therm Anal Calorim. 2012;107:305–11. doi:10.1007/s10973-011-1451-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, L.C., de Carvalho, M.A., Marins, A.A.L. et al. Influence of ethyl alcohol in the preparation, morphology and properties of compound DAS–Eu3+ and its thermal degradation products. J Therm Anal Calorim 114, 537–547 (2013). https://doi.org/10.1007/s10973-013-2958-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2958-x