Abstract
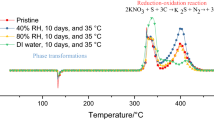
The objective of this article is to generate thermal decomposition data on fireworks tip mixture, a mixture used to coat the tip of fireworks, for easy ignition. This mixture has reportedly involved in triggering many accidents in fireworks industry. Different quantities of water were added to the mixture and its thermal characteristics were studied. Differential scanning calorimeter was used for screening tests and accelerating rate calorimeter was used for detailed studies in adiabatic and isothermal modes. The self-heat rate data obtained showed onset temperature for different quantity of water, at a range of 80–170 °C. The mixture with 40 % water wt/wt had onset at 80 °C in adiabatic mode. The same mixture on isoaging at 40 °C exhibited exothermic characteristics with a substantial rise in system pressure (57 bar). The heats of exothermic decomposition and Arrhenius kinetics were also computed.










Similar content being viewed by others
Abbreviations
- KNO3 :
-
Potassium nitrate
- BaNO3 :
-
Barium nitrate
- Al:
-
Aluminum
- C :
-
Concentration
- T :
-
Temperature/°C
- T 0 :
-
Initial temperature/°C
- T F :
-
Final temperature/°C
- ∆H r :
-
Heat of reaction/J g−1
- ϕ:
-
Thermal inertia
- ΔT :
-
Temperature difference/°C
- ΔT ad :
-
Corrected temperature difference/°C
- m S :
-
Mass of sample/g
- m B :
-
Mass of bomb/g
- k :
-
Rate coefficient
- m T :
-
Rate of temperature increase/°C min−1
- k* :
-
Pseudo rate constant
- E a :
-
Activation energy/kC mol−1
- R :
-
Universal gas constant
- A :
-
Pre-exponential factor/s−1
- \( \bar{C}_{\text{p}} \) :
-
Average heat capacity/J g−1 K−1
- \( \bar{C}_{\text{ps}} \) :
-
Average heat capacity of sample/J g−1 K−1
- \( \bar{C}_{\text{pB}} \) :
-
Average heat capacity of bomb/J g−1 K−1
References
Sivapirakasam SP, Surianarayanan M, Venkataratnam GS, Nagaraj P. Hazard evaluation technique for firework compositions. Bhubaneswar: Indian Chemical Engineering Congress; 2003. p. 126.
Sivapirakasam SP, Surianarayanan M, Venkataratnam GS, Nagaraj P. Impact sensitiveness analysis of pyrotechnic flash compositions. J Pyrotechnol. 2005;21:51–8.
Herder G, Weterings FP, de Klerk WPC. Mechanical analysis of rocket propellants. J Therm Anal Calorim. 2003;72:921–9.
Sridhar VP, Surianarayanan M, Sivapirakasam SP, Mandal AB. Adiabatic thermokinetics and process safety of pyrotechnic mixtures atom bomb, Chinese, and palm leaf crackers. J Therm Anal Calorim. 2012;109:1387–95.
Sivapirakasam SP, Surianarayanan M, Chandrasekran F. Thermal characterization of pyrotechnic flash compositions. Sci Technol Energ Mater. 2010;71:1–6.
Hou HY, Duh YS, Lee WL, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;95(2):541–5.
Roy M, Dave P, Barbar SK, Jangid S, Phase DM, Awasthi AM. X-ray, SEM and DSC studies of ferroelectric Pb1-xBaxTiO3 ceramics. J Therm Anal Calorim. 2010;101:833–7.
Simoes RD, Perez MAR, De Saja JA, Constantino CJL. Thermomechanical characterization of PVDF and P(VDF–TrFE) blends containing corn starch and natural rubber. J Therm Anal Calorim. 2010;99:621–9.
Iizuka Y, Surianarayanan M. Comprehensive kinetic model for adiabatic decomposition of di-tert-butyl peroxide using batch CAD. Ind Eng Chem Res. 2003;42:2987–95.
Smitha VS, Surianarayanan M, Seshadri H, Lakshman NV, Mandal AB. Reactive thermal hazards of tributyl phosphate with nitric acid. Ind Eng Chem Res. 2012;51(21):7205–10.
Jinyang Y, Liping C, Jinhua P. Thermal hazard research of smokeless fireworks. J Therm Anal Calorim. 2012;109:1151–6.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimetry. Thermochem Acta. 1980;37:1–30.
ESARC. The accelerating rate calorimeter operations manual. Thermal hazard technology, version 1.0. Bordeaux: ESARC, October 2006.
Smitha VS, Surianarayanan M, Seshadri H, Mandal AB. Thermal behavior pattern of tributyl phosphate under adiabatic conditions. J Therm Anal Calorim. 2012. doi:10.1007/s10973-012-2197-6.
Acknowledgements
The authors are thankful to Prof NR Rajagopal for his encouragement. One of the authors Sridhar VP thanks CSIR, New Delhi for SRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sridhar, V.P., Surianarayanan, M., Sivapirakasam, S.P. et al. Accelerating rate calorimeter studies of water-induced thermal hazards of fireworks tip mixture. J Therm Anal Calorim 112, 1335–1341 (2013). https://doi.org/10.1007/s10973-012-2716-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2716-5