Abstract
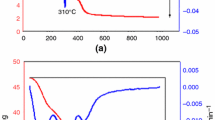
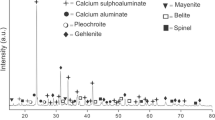
This paper represents a laboratory study on the acid resistance of hardened ordinary Portland cement (OPC) and blended OPC pastes at two different curing temperatures. The blended materials used are rice husk ash (RHA) and cement kiln dust (CKD). The blended cement pastes were prepared using a water/solid (W/S) ratio of 0.3. The effects of immersion in deionized water (pH 7) and sulfuric acid solutions (pH 1, 2 and 3) at two temperatures (20 and 50 °C) on the compressive strength and phase composition of the various hardened blended cement pastes were studied. The results of compressive strength revealed that the increase of curing temperature from 20 to 50 °C resulted in increase the reduction of compressive strength due to acid attack up 2 months, but the resistance to sulfuric acid attack increases after that time due to the formation of crystalline calcium silicate hydrates (CSH) which have higher resistance to acid attack than the amorphous CSH formed at the early ages of hydration. The presence of RHA and CKD improves the resistance to sulfuric acid attack at both curing conditions. From the results of X-ray diffraction analysis and differential scanning calorimetric technique curves, the main hydration products identified are CSH, portlandite, and calcium sulfoaluminate hydrates.










Similar content being viewed by others
References
Vladimir Z, Adolf B. Acid attack of cement based materials—a review, part 1: principle of acidic attack. Constr Build Mater. 2001;15:331–40.
ACI Committee 201. Quid to durable concrete American Concrete Institute, Report of ACI Committee. Detroit: ACI 201.2R-77, DETER 1982;37.
Jahani F, Devinny J, Mansfeld F, Rosen IG, Sun Z, Wang C. Investigations of sulfuric acid corrosion of concrete. I: modeling and chemical observations. J Environ Eng. 2001;127:572–9.
Jahani F, Devinny J, Mansfeld F, Rosen IG, Sun Z, Wang C. Investigations of sulfuric acid corrosion of concrete. II: electrochemical and visual observations. J Environ Eng. 2001;127:580–4.
Tulliani JM, Montanaro L, Negro A, Collepardi M. Sulfate attack of concrete building foundations induced by sewage waters. Cem Concr Res. 2002;32:843–9.
Torres SM, Sharp JH, Swamy RN, Lynsdale CJ, Huntley SA. Long term durability of Portland-limestone cement mortars exposed to magnesium sulfate attack. Cem Concr Res. 2003;25:947–54.
Vipulanandan C, Liu J. Glass–fiber mat-reinforced epoxy coating for concrete in sulfuric acid environment. Cem Concr Res. 2002;32:205–10.
Girardi F, Vaona W, Di Maggio R. Resistance of different types of concretes to cyclic sulfuric acid and sodium sulfate attack. Cem Concr Comp. 2010;32:595–602.
Delagrave A, Pigeon M, Revertégat E. Influence of chloride ions and pH level on the durability of high performance cement pastes. Cem Concr Res. 1994;24:1433–43.
Allahverdi A, František Š. Gypsum -free Portland cement, an alkali-activated material suitable for acid corrosion protection. Proc. 3rd Int. conference on alkali activated materials, Prague, Czech Republic 2007; p. 39–53, June 21–22.
Allahverdi A, Škvára F. Acidic corrosion of hydrated cement based materials, part 1; mechanism of the phenomenon. Ceram Silik. 2000;44(3):114–20.
Kosmatka SH, Kerkhoff B, Panarese WC. Design and control of concrete mixtures. Skokie, IL: Portland Cement Association; 2003.
De Belie N, Debruyckere M, Van Nieuwenburg D, De Blaere B. Attack of concrete floors in pig houses by feed acids: influence of fly ash addition and cement-bound. Surf Layer. 1997;68(2):101–8.
Zhen-Tian C, Xiu-Jiang S, Robert M, Marton M. Using limestone aggregates and different cements for enhancing resistance of concrete to sulphuric acid attack. Cem Concr Res. 2005;35:1486–94.
Durning TA, Hicks MC. Using microsilica to increase concrete’s resistance to aggressive chemicals. Concr Int. 1991;13(3):42–8.
Kazuyuk T, Mitsunor K. Effects of fly ash and silica fume on the resistance of mortar to sulphuric acid and sulphate attack. Cem Concr Res. 1994;24(2):361–70.
Mehta PK. Studies of chemical resistance of low water/cement ratio concretes. Cem Concr Res. 1985;15(6):969–78.
Fattuhi NI, Hughes BP. Ordinary Portland cement mixes with selected admixtures subjected to sulphuric acid attack. ACI Mater J. 1988;85(6):512–8.
Caballero CE, Sanchez E, Cano U, Gonzalez JG, Castano V. On the effect of fly ash on the corrosion properties of reinforced mortars. Corros Rev. 2000;18(2–3):105–12.
Tamimi AK. High performance concrete mix for an optimum protection in acidic conditions. Mater Struct. 1997;30:188–91.
Cook DJ. Rice husk ash concrete technology and design, cement replacement materials, vol. 3. London: Surrey University Press; 1986. p. 171–196.
Paya J, Monzo J, Borrachero MV, Mellado A, OrdonÄez LM. Determination of amorphous silica in rice husk ash by a rapid analytical method. Cem Concr Res. 2001;31:227–31.
Amin MS, Habib AO, Abo-El-Enein SA. Hydrothermal characteristics of high slag cement pastes made with and without silica sand. Adv Cem Res. 2012;24(1):23–31.
Kondo R, Abo-El-Enein SA, Daimon M. Kinetics and mechanism of hydrothermal reaction of granulated blast furnace slag. Bull Chem Soc Jpn. 1975;48:222–6.
Heikal M, Morsy MS, El-Shimy E, Abo-El-Enein SA. L’industria italiana del Cemento. 2004;800:614–24.
El-Gamal SMA, Hashem FS, Amin MS. Thermal resistance of hardened cement pastes containing vermiculite and expanded vermiculite. J Therm Anal Calorim. 2011. doi:10-1007/s10973-011-1680-9.
Morsy MS, Galal AF, Abo-El-Enein SA. Effect of temperature on phase composition and microstructure of artificial pozzolana cement pastes containing burnt kaolinite clay. Cem Concr Res. 1998;28(8):1157–63.
Hidalgo A, Garcia JL, Alonso MC, Fernandez L, Andrade C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J Therm Anal Calorim. 2009;96(2):335–45.
Chaipanich A, Nochaiya T. Thermal analysis and microstructure of Portland cement–fly ash–silica fume pastes. J Therm Anal Calorim. 2010;99:487–93.
Nochaiya T, Wongkeo W, Pimraksa K, Chaipanich A. Microstructural, physical, and thermal analysis of Portland cement–fly ash–calcium hydroxide blended pastes. J Therm Anal Calorim. 2010;100:101–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashem, F.S., Amin, M.S. & El-Gamal, S.M.A. Improvement of acid resistance of Portland cement pastes using rice husk ash and cement kiln dust as additives. J Therm Anal Calorim 111, 1391–1398 (2013). https://doi.org/10.1007/s10973-012-2458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2458-4