Abstract
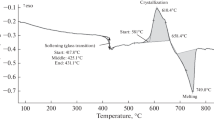
Calcium sulfate dihydrate has been widely characterized by both differential scanning calorimetry and thermogravimetry (TG). Two dehydration processes were reported to be partially overlapping. High resolution TG and water vapor self generated atmosphere pin-hole lid strategies were used to increase the resolution of both dehydration processes. In this study, isobaric experiments were carried out in a pressure differential scanning calorimetry cell. The approach consisted in combining the pin-hole lid with different pressures with nitrogen atmosphere. Resolution was improved at moderately low pressures. At higher pressures other processes were observed.







Similar content being viewed by others
References
Groves AW. Gypsum and anhydrite. London: Her Majesty’s Stationery Office; 1958. p. 108.
Taylor HFW. Cement chemistry. London: Academic Press; 1990. p. 233.
Belmiloudi A, Le Meur G. Mathematical and numerical analysis of dehydration of gypsum plasterboards exposed to fire. Appl Math Comput. 2005;163:1023–41.
Ramachandran VS. Applications of differential thermal analysis in cement chemistry. New York: Chemical Publishing Co Inc.; 1969. p. 251–270.
Lou W, Guan B, Wu Z. Dehydration behavior of FGD gypsum by simultaneous TG and DSC analysis. J Therm Anal Calorim. 2011;104:661–9.
Lopez-Beceiro J, Pascual-Cosp J, Artiaga R, Tarrio-Saavedra J, Naya S. Thermal characterization of ammonium alum. J Therm Anal Calorim. 2011;104:127–30.
Khalil AA, Hussein AT, Gad GM. On the thermochemistry of gypsum. J Appl Chem Biotech. 1971;21:314–6.
Molony B, Ridge MJ. Kinetics of the dehydration of calcium sulphate dihydrate in vacuo. Aust J Chem. 1968;21:1963–5.
Ball MC, Norwood LS. Studies in the system calcium sulphate–water. Part I. Kinetics of dehydration of calcium sulphate dihydrate. J Chem Soc A. 1969;0:1633–7.
Fatu D. Kinetics of gypsum dehydration. J Therm Anal Cal. 2001;65:213–20.
Lehman H, Rieke K. Investigations of the system CaSO4–H2O under special considerations of material and experimental parameters by differential thermal analysis. Proceedings of the 4th international conference on thermal analysis. Budapest; 1975;1:573–83.
Dantas HF, Mendes RAS, Pinho RD, Soledade LEB, Paskocimas CA, Lira BB, Schwartz MOE, Souza AG, Santos IMG. Characterization of gypsum using TMDSC. J Therm Anal Cal. 2007;87:691–5.
Tydlitát V, Medveď I, Černý R. Determination of a partial phase composition in calcined gypsum by calorimetric analysis of hydration kinetics. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1334-y.
Clifton JR. Thermal analysis of calcium sulfate dihydrate and supposed a and b forms of calcium sulfate from 25 to 500 °C. J Res Natl Bur Stand-A. 1972;76A:41–9.
Comodi P, Kurnosov A, Nazzareni S, Dubrovinsky L. The dehydration process of gypsum under high pressure. Phys Chem Miner. 2012;39:65–71.
Ramachandran VS. Concrete admixtures handbook. New Delhi: Standard Publishers; 2002.
Ramachandran VS. Handbook of thermal analysis of construction materials. Norwich: Noyes Publications/William Andrew Pub; 2003.
Suwardie J, Artiaga R, Barbadillo F. Simultaneous thermal analysis of hexahydrophtalic anhydride. Thermochim Acta. 2002;392:289–94.
Elliot C. Plaster of Paris technology. Chem Trade J. 1923;72:725–6.
Acknowledgements
This study was partially funded by the Spanish Ministerio de Educacion y Ciencia MTM2008-00166 and MAT2010-21342-C02-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Beceiro, J., Gracia-Fernández, C., Tarrío-Saavedra, J. et al. Study of gypsum by PDSC. J Therm Anal Calorim 109, 1177–1183 (2012). https://doi.org/10.1007/s10973-012-2335-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2335-1