Abstract
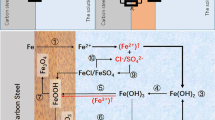
The influence of different ions NO3 − and SO4 2− on the carbon steel corrosion in ammonium chloride was investigated using mass loss measurements and potentiodynamic polarization. Corrosion products were analyzed using X-ray photoelectron spectroscopy (XPS) and simultaneous thermal and differential scanning calorimetry (TG/DSC). XPS analysis shows that the main product of corrosion is a non-stoichiometric Fe3+ oxyhydroxide, consisting of a mixture of FeO(OH) and FeO(OH) containing inclusions of these anions, species such as Fe3+O(OH,Cl−); Fe3+O(OH,SO4 2−); and Fe3+O(OH,NO3 −). TG/DSC confirms the decomposition of the rusty products formed by chemical corrosion, compounds like Fe3+ oxyhydroxides, with β-FeOOH as the major phase, crystal structure of which may contain Cl−, NO3 −, and SO4 2−—e.g., akaganeite [Fe3+O(OH,A)].



Similar content being viewed by others
References
Balek V, Subrt J. Thermal behaviour of iron(III) oxide hydroxides. Pure Appl Chem. 1995;67:1839–42.
Samide A, Bibicu I, Rogalski M, Preda M. Surface study of the corrosion of carbon steel in solutions of ammonium salts using Mossbauer spectrometry. J Radioanal Nucl Chem. 2004;261:593–9.
Refait P, Abdelmoula M, Génin JMR, Sabot R. Green rusts in electrochemical and microbially influenced corrosion of steel. Compt Rend Geosci. 2006;338:476–87.
Santana Rodríguez JJ, Santana Hernández FJ, González González JE. XRD and SEM studies of the layer of corrosion products for carbon steel in various different environments in the province of Las Palmas (The Canary Islands, Spain). Corros Sci. 2002;44:2425–38.
Santana Rodríguez JJ, Santana Hernández FJ, González González JE. Mathematical and electro-chemical characterisation of the layer of corrosion products on carbon steel in various environments. Corros Sci. 2002;44:2597–610.
Zhang Q, Wang R, Kato M, Nakasa K. Observation by atomic force microscope of corrosion product during pitting corrosion on SUS304 stainless steel. Script Mater. 2005;52:227–30.
Tamura H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros Sci. 2008;50:1872–83.
Wang Z, Moore RC, Felmy AR, Mason MJ, Kukkadapu RK. A study of the corrosion products of mild steel in high ionic strength. Waste Manage. 2001;50:1872–83.
Przepiera K, Przepiera A. Kinetics of thermal transformations of pricipitated magnetite and goethite. J Therm Anal Calorim. 2001;65:497–503.
Dinesen AR, Pedersen CT, Bender Koch C. The thermal conversion of lepidocrocite (γ-FeOOH) revised. J Therm Anal Calorim. 2001;64:1303–10.
Ichiyanagi Y, Kimishima Y. Structural, magnetic and thermal characterizations of Fe2O3 nanoparticle systems. J Therm Anal Calorim. 2002;69:919–23.
Samide A, Preda M, Chirita P, Rusu O. The effect of the CO3 2− anion on the corrosion of carbon steel in an ammonium chloride solution. Rev Chim. 2004;55:1022–9.
Refaey SAM. Inhibition of steel pitting corrosion in HCl by some inorganic anions. Appl Surf Sci. 2005;240:396–404.
Abd El Haleem SM, Abd El Wanees S, Abd El Aal EE, Diab A. Environmental factors affecting the corrosion behavior of reinforcing steel. IV. Variation in the pitting corrosion current in relation to the concentration of the aggressive and the inhibitive anions. Corros Sci. 2010;52:1675–83.
Refaey SAM, Taha F, Abd El-Malak AM. Corrosion and inhibition of stainless steel pitting corrosion in alkaline medium and the effect of Cl− and Br− anions. Appl Surf Sci. 2005;242:114–20.
Takasaki S, Yamada Y. Effects of temperature and aggressive anions on corrosion of carbon steel in potable water. Corros Sci. 2007;49:240–7.
Vijayan CP, Le HH, Galibois A, Ghali E. Influence of some anions on the stress corrosion of low alloy steels in aqueous solutions. Surf Coat Technol. 1988;34:561–72.
Deyab MA, Abd El-Rehim SS. Inhibitory effect of tungstate, molybdate and nitrite ions on the carbon steel pitting corrosion in alkaline formation water containing Cl− ion. Electrochim Acta. 2007;53:1754–60.
El-Naggar MM. Effects of Cl−, NO3 − and SO4 2− anions on the anodic behavior of carbon steel in deaerated 0.5 M NaHCO3 solutions. Appl Surf Sci. 2006;252:6179–94.
Zhao JM, Zuo Y. The effects of molybdate and dichromate anions on pit propagation of mild steel in bicarbonate solution containing Cl−. Corros Sci. 2002;44:2119–30.
Ma Y, Li Y, Wang F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros Sci. 2009;51:997–1006.
Deyab MA. Effect of cationic surfactant and inorganic anions on the electrochemical behavior of carbon steel in formation water. Corros Sci. 2007;49:2315–28.
Tang J, Shao Y, Zhang T, Meng G, Wang F. Corrosion behaviour of carbon steel in different concentrations of HCl solutions containing H2S at 90 °C. Corros Sci. 2011;53:1715–23.
Refaey SAM, Abd El-Rehim SS, Taha F, Saleh MB, Ahmed RA. Inhibition of chloride localized corrosion of mild steel by PO4 3−, CrO4 2−, MoO4 2−, and NO2 − anions. Appl Surf Sci. 2000;158:190–6.
Grosvenor AP, Kobe BA, Biesinger MC, McIntyre NS. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf Interface Anal. 2004;36:1564–74.
Dupin JC, Gonbeau D, Vinatier P, Levasseur A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys Chem Chem Phys. 2000;2:1319–24.
Yi ZA, Xu YY, Zhu LP, Dong HB, Zhu BK. Hydrophilic modification of peask porous membranes via aqueous surface-initiated atom transfer radical polymerization. Chinese J Polym Sci. 2009;27:695–702.
Vinnichenko M, Chevolleau T, Pham MT, Poperenko L, Maitz MF. Spectroellipsometric, AFM and XPS probing of stainless steel surfaces subjected to biological influences. Appl Surf Sci. 2002;201:41–50.
Shah Singh S, Kodama H. Effect of the presence of aluminum ions in iron solutions on the formation of iron oxyhydroxides (FeOOH) at room temperature under acidic environment. Clay Clay Miner. 1994;42:606–13.
Gialanella S, Girardi F, Ischia G, Lonardelli I, Mattarelli M, Montagna M. On the goethite to hematite phase transformation. J Therm Anal Calorim. 2010;102:867–73.
Walter D, Buxbaum G, Laqua W. The mechanism of the thermal transformation from goethite to hematite. J Therm Anal Calorim. 2001;63:733–48.
Petkova V, Pelovski Y. Comparative DSC study on thermal decomposition of iron sulphates. J Therm Anal Calorim. 2008;93:847–52.
Acknowledgements
This study was supported by CNCSIS—UEFISCSU, under project number PNII—IDEI 422/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tutunaru, B., Samide, A. & Negrila, C. Thermal analysis of corrosion products formed on carbon steel in ammonium chloride solution. J Therm Anal Calorim 111, 1149–1154 (2013). https://doi.org/10.1007/s10973-011-2187-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2187-0