Abstract
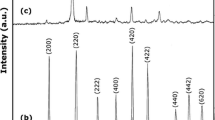
This paper presents a study regarding the obtaining of NiCr2O4 by two new unconventional synthesis methods: (i) the first method is based on the formation of Cr(III) and Ni(II) carboxylate-type precursors in the redox reaction between the nitrate ion and 1,3-propanediol. The thermal decomposition of these complex combinations, at ~300 °C, leads to an oxide mixture of Cr2O3+x and NiO, with advanced homogeneity, small particles and high reactivity. On heating this mixture at 500 °C, Cr2O3 reacts with NiO to form NiCr2O4, which was evidenced by FT-IR and X-ray diffractometry (XRD) analysis; (ii) the second method starts from a mechanical mixture of (NH4)2Cr2O7 and Ni(NO3)2·6H2O. On heating this mixture, a violent decomposition at 240 °C with formation of an oxides mixture (Cr2O3 + CrO3) and NiO takes place. On thermal treatment up to 500 °C, an intermediary phase NiCrO4 is formed, which by decomposition at ~700 °C leads to NiCr2O4, evidenced by FT-IR and XRD analysis. NiCr2O4 is formed, in both cases, starting with a temperature higher than 400 °C, when the non-stoichiometric chromium oxide (Cr2O3+x ) loses the oxygen excess and turns to stoichiometric chromium oxide (Cr2O3), which further reacts with NiO.








Similar content being viewed by others
Abbreviations
- 1, 3PG:
-
1, 3-Propanediol
References
Jebarathinam NJ, Eswaramoorthy M, Krishnasamy V. Dehydrogenation of ethylbenzene over spinel oxides. Bull Chem Soc Japan. 1994;67:3334–8.
Sloczynski J, Ziolkowski J, Grzybowska B, Grabowski R, Jachewicz D, Wcislo K, Gengembre L. Oxidative dehydrogenation of propane on Ni x Mg1−x Al2O4 and NiCr2O4 spinels. J Catal. 1999;187:410–8.
Honeybourne CL, Rasheed RK. Nitrogen dioxide and volatile sulfide sensing properties of copper, zinc and nickel chromite. J Mat Chem. 1996;6:277–83.
Strawbridge A, Stott FH, Wood GC. The formation and incorporation into the scale of internal oxides developed during the high-temperature oxidation of dilute nickel-base alloys. Corros Sci. 1993;35:852–5.
Mančić L, Marinković ZV, Vulić P, Milošević O. The synthesis–structure relationship in the ZnO–Cr2O3 system. Sci Sinter. 2004;36:189–96.
Szczygiel I, Winiarska K. Low-temperature synthesis and characterization of the Mn–Zn ferrite. J Therm Anal Calorim. 2011;104:577–83.
Souaya ER, Ismail EH, Mohamed AA, Milad NE. Preparation, characterization and thermal studies of some transition metal ternary complexes. J Therm Anal Calorim. 2009;95:253–8.
Stefanescu M, Stefanescu O, Stoia M, Lazau C. Thermal decomposition of some metal-organic precursors: Fe2O3 nanoparticles. J Therm Anal Calorim. 2007;88(1):27–32.
Suryanarayana C, Grant Norton M. X-ray diffraction: a practical approach. New York: Plenum Press; 1998.
Stefanescu M, Barbu M, Vlase T, Barvinschi P, Barbu-Tudoran L, Stoia M. Novel low temperature synthesis method for nanocrystalline zinc and magnesium chromites. Thermochim Acta. 2011. doi:10.1016/j.tca.2011.09.005.
Stefanescu M, Sasca V, Birzescu M. Thermal behaviour of the homopolynuclear glyoxylate complex combinations with Cu(II) and Cr(III). J Therm Anal Calorim. 2003;72(2):515–24.
Stefanescu M, Sasca V, Birzescu M. Studies on the thermal decompositions of heteropolynuclear glyoxylates of Cr(III) and Cu(II). J Therm Anal Calorim. 1999;56(2):569–78.
Pocol V, Patron L, Carp O, Brezeanu M, Segal E, Stanica N. Some polynuclear coordination compounds precursor of chromites synthesis, physicochemical characterization and thermal stability. J Therm Anal Calorim. 1999;55:143–54.
Terada M, Maekawa T. The infrared absorption spectra of several chromites. Trans JIM. 1964;5:205–6.
Joint Committee on powder diffraction standards-International Center for Diffraction Data, Swarthmore; 1993;23-0432.
Holman HYN, Perry DL, Martin MC, Lamble GM, McKinney WR, Hunter-Cevera JC. Real-time characterization on biogeochemical reduction of Cr(VI) on basalt surfaces by SR-FTIR imaging. Geomicrobiol J. 1999;16:307–24.
Acknowledgements
This work was partially supported by the strategic grant POSDRU/88/1.5/S/50783, Project ID50783 (2009), co-financed by the European Social Fund–Investing in People, within the Sectoral Operational Programme Human Resources Development 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ştefănescu, M., Barbu, M., Barvinschi, P. et al. The obtaining of NiCr2O4 nanoparticles by unconventional synthesis methods. J Therm Anal Calorim 111, 1121–1127 (2013). https://doi.org/10.1007/s10973-011-2177-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2177-2