Abstract
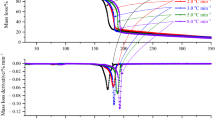
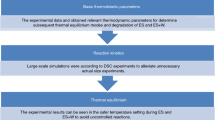
The heat release behaviors of several chemical compounds were detected with C80 calorimeter. Furthermore, the thermodynamic and kinetic parameters of the chemicals were obtained based on the experimental data. Chemical thermal risk was simultaneously graded with combined use of instantaneous power density and the classification standard of thermal instability which was offered by the standard system for the identification of the hazards of materials for emergency response (NFPA704). The results show that the grading standard of thermal risk based on NFPA704 was reasonable and can be used as a preliminary thermal risk assessment for reaction hazards.



Similar content being viewed by others
Abbreviations
- C 0 :
-
Initial reaction rate of reactant
- Rate:
-
Reaction rate of reactant (g mL−1 s−1)
- M 0 :
-
Initial mass of reactant
- M :
-
Mass of reactant at time t
- A :
-
Pre-exponential factor of Arrhenius equation (s−1)
- E a :
-
Activation energy (kJ mol−1)
- ΔH :
-
Reaction heat of unit reactant (kJ mol−1)
- q G :
-
Heat release (kJ mol−1)
- t :
-
Reaction time (min)
- T :
-
Temperature of system at time t (K)
- T o,s :
-
Onset exothermic temperature
- n :
-
Reaction order
- k :
-
Constant of reaction rate
- R :
-
Gas constant (8.31415 J mol−1 K−1)
- dH/dt :
-
Overall heat flow (W)
- dT/dt :
-
Self-heat rate (K s−1)
References
State Administration of Work Safety Supervision of China. Analysis on typical serious accidents caused by hazardous chemicals at home and abroad. Qingdao: Bureau of Press and Publication of Qingdao, China; 2002.
Transportation Research Board 2009 Executive Committee. Hazardous Materials Trans-portation Incident Data for Root Cause Analysis, Washington, DC, 2009.
Recommendations on the transport of dangerous goods, model regulations, 16th rev. ed. United Nations, New York, 2009.
Tseng JM, Liu MY, Chen SL, Hwang WT, Gupta JP, Shu CM. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III tests. J Therm Anal Calorim. 2009;96:789–93.
European agreement concerning the international carriage of dangerous goods by road (ADR), United Nations, New York, 2009.
Ashar SM, Harrison BK. CHETAH version 7.2 The ASTM computer program for chemical thermodynamic and energy release evaluation. West Conshohocken: ASTM Subcommittee; 1998.
Wang QS, Rogers WJ, Mannan MS. Thermal risk assessment and rankings for reaction hazards in process safety. J Therm Anal Calorim. 2009;98:225–33.
Stoessel F, Ubrich O. Safety assessment and optimization of semi-batch reactions by calorimetry. J Therm Anal Calorim. 2001;64:61–74.
Iwata Y, Momota M, Koseki H. Thermal risk evaluation of organic peroxide by automatic pressure tracking adiabatic calorimeter. J Therm Anal Calorim. 2006;85:617–22.
Wei C, Rogers WJ, Mannan MS. Layer of protection analysis for reactive chemical risk assessment. J Hazard Mater. 2008;159:19–24.
Chou YP, Hou HY, Chang RH, You ML, Peng JY. Thermal decomposition of cumene hydroperoxide in the presence of three incompatible substances by isothermal microcalorimetry and high performance liquid chromatography. J Therm Anal Calorim. 2009;96:771–5.
Ding SD, Bai CY, Liu ZP, Wang YZ. Enhanced thermal stability of poly(p-dioxanone) in melt by adding an end-capping reagent. J Therm Anal Calorim. 2008;94:89–95.
Lin CP, Tseng JM, Chang YM, Liu SH, Shu CM. Modeling liquid thermal explosion reactor containing tert-butyl peroxybenzoate. J Therm Anal Calorim. 2010;102:587–95.
Lin CP, Chang CP, Chou YC, Chu YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Saraf SR, Rogers WJ, Mannan MS. Classifying reactive chemicals. Chem Eng Prog. 2004;100(3):34–7.
NFPA704, Standard System for the Identification of the Hazards of Materials for Emergency Response. National Fire Protection Association, 2001. http://www.nfpa.org.
Guidelines for Safe Storage and Handling of Reactive Materials (CCPS 1995b). New York: Center for Chemical Process Safety of the American Institute of Chemical Engineers; 1995.
Cardillo P, Gigante L, Lunghi A, Zanirato P. Revisiting the thermal decomposition of five ortho-substituted phenyl azides by calorimetric techniques. J Therm Anal Calorim. 2010;100:191–8.
Lin CP, Shu CM. A comparison of thermal decomposition energy and nitrogen content of nitrocellulose in non-fat process of linters by DSC and EA. J Therm Anal Calorim. 2009;95:547–52.
Sun JH, Li YF, Hasegawa K. A study of self-accelerating decomposition temperature (SADT) using reaction calorimetry. J Loss Prev Process Ind. 2001;14:331–6.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Sun JH, Ding H. Evaluation on thermal risk of chemicals. Beijing: Science Press; 2005.
Robinson LA. Introduction to Part 1 of the special series on risk regulation. Risk Anal. 2010;31(6):881–3.
Lin WH, Wu SH, Shiu GY, Shieh SS, Shu CM. Self-accelerating decomposition temperature (SADT) calculation of methyl ethyl ketone peroxide using an adiabatic calorimeter and model. J Therm Anal Calorim. 2009;95(2):645–51.
Tuma LD. Identification of potential thermal runaways using small scale thermal analytical techniques. J Therm Anal. 1997;49:1689–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, D., Xie, C., Zhang, F. et al. Thermal risk assessment and grading of chemicals with instantaneous power density. J Therm Anal Calorim 109, 1373–1377 (2012). https://doi.org/10.1007/s10973-011-1836-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1836-7