Abstract
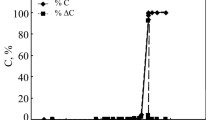
Non-isothermal oxidation kinetics of single- and multi-walled carbon nanotubes (CNTs) have been studied using thermogravimetry up to 1273 K in ambient using multiple heating rates. One single heating rate based model-fitting technique and four multiple heating rates based model-free isoconversional methods were used for this purpose. Depending on nanotube structure and impurity content, average activation energy (E a), pre-exponential factor (A), reaction order (n), and degradation mechanism changed considerably. For multi-walled CNTs, E a and A evaluated using model-fitting technique were ranged from 142.31 to 178.19 kJ mol−1, respectively, and from 1.71 × 105 to 5.81 × 107 s−1, respectively, whereas, E a for single-walled CNTs ranged from 83.84 to 148.68 kJ mol−1 and A from 2.55 × 102 to 1.18 × 107 s−1. Although, irrespective of CNT type, the model-fitting method resulted in a single kinetic triplet i.e., E a, A, and reaction mechanism, model-free isoconversional methods suggested that thermal oxidation of these nanotubes could be either a simple single-step mechanism with almost constant activation energy throughout the reaction span or a complex process involving multiple mechanisms that offered varying E a with extent of conversion. Criado method was employed to predict degradation mechanism(s) of these CNTs.









Similar content being viewed by others
References
Terrones M. Science and technology of the twenty-first century: synthesis, properties, and applications of carbon nanotubes. Annu Rev Mater Res. 2003;33:419–501.
Breuer O, Sundararaj U. Big returns from small fibers: a review of polymer/carbon nanotube composites. Polym Compos. 2004;25:630–41.
Samal SS, Bal S. Carbon nanotube reinforced ceramic matrix composites—a review. J Miner Mater Character Eng. 2008;7(4):355–70.
Bakshi SR, Lahiri D, Agarwal A. Carbon nanotube reinforced metal matrix composites–a review. Int Mater Rev. 2010;55(1):41–64.
Song W-l, Cao M-S, Hou Z-l, Yuan J, Fang X-Y. High-temperature microwave absorption and evolutionary behavior of multiwalled carbon nanotube nanocomposite. Scr Mater. 2009;61:201–4.
Chen Z-K, Yang J-P, Ni Q-Q, Fu S-Y, Huang Y-G. Reinforcement of epoxy resins with multi-walled carbon nanotubes for enhancing cryogenic mechanical properties. Polymer. 2009;50:4753–9.
Illeková E, Csomorová K. Kinetics of oxidation in various forms of carbon. J Therm Anal Calorim. 2005;80:103–8.
Brukh R, Mitra S. Kinetics of carbon nanotube oxidation. J Mater Chem. 2007;17:619–23.
Vignes A, Dufaud O, Perrin L, Thomas D, Bouillard J, Janès A, et al. Thermal ignition and self-heating of carbon nanotubes: from thermokinetic study to process safety. Chem Eng Sci. 2009;64:4210–21.
Sarkar S, Das PK, Bysakh S, Dasgupta K. Evaluation of thermal stability of commercial multiwalled carbon nanotubes. First Asian Carbon Conference, New Delhi, 2009.
Sarkar S, Das PK, Bysakh S. Effect of heat treatment on morphology and thermal decomposition kinetics of multiwalled carbon nanotubes. Mater Chem Phys. 2011;125:161–7.
Al-Othman AA, Al-Farhan KA, Mahfouz RM. Kinetic analysis of nonisothermal decomposition of (Mg5(CO3)4(OH)2·4H2O/5Cr2O3) crystalline mixture. J King Saud Univ (Sci). 2009;21:133–43.
Janković B, Mentus S, Jelic D. A kinetic study of non-isothermal decomposition process of anhydrous nickel nitrate under air atmosphere. Physica B. 2009;404:2263–9.
Boonchom B, Danvirutai C, Thongkam M. Non-isothermal decomposition kinetics of synthetic serrabrancaite (MnPO4·H2O) precursor in N2 atmosphere. J Therm Anal Calorim. 2010;99:357–62.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4·3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98:863–71.
Jiao-qiang Z, Hong-xu G, Li-hong S, Rong-zu H, Feng-qi Z, Bo-zhou W. Non-isothermal thermal decomposition reaction kinetics of 2-nitroimino-5-nitro-hexahydro-1,3,5-triazine (NNHT). J Hazard Mater. 2009;167:205–8.
Wang Y-F, Liu J-F, Xian H-D, Zhao G-L. Synthesis, crystal structure, and kinetics of the thermal decomposition of the nickel(ii) complex of the Schiff base 2-[(4-Methylphenylimino)methyl]-6-methoxyphenol. Molecules. 2009;14:2582–93.
Chen Y, Wang Q. Thermal oxidative degradation kinetics of flame-retarded polypropylene with intumescent flame-retardant master batches in situ prepared in twin-screw extruder. Polym Degrad Stabil. 2007;92:280–91.
Doğan F, Kaya I, Bilici A. Non-isothermal degradation kinetics of poly (2,2′-dihydroxybiphenyl). Polym Bull. 2009;63:267–82.
Doğan F, Kaya I, Bilici A, Saçak M. Thermal decomposition kinetics of azomethine oligomer and its some metal complexes. J Appl Polym Sci. 2010;118:547–56.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, et al. Computational aspects of kinetic analysis Part A: the ICTAC kinetics project-data. Thermochim Acta. 2000;355:125–43.
Vyazovkin S. Computational aspects of kinetic analysis. Part C. The ICTAC kinetics project-the light at the end of the tunnel? Thermochim Acta. 2000;355:155–63.
Vyazovkin S. Reply to “What is meant by the term ‘variable activation energy’ when applied in the kinetics analyses of solid state decompositions (crystolysis reactions)?” Thermochim Acta. 2003;397:269–71.
Pratap A, Rao TLS, Lad KN, Dhurandhar HD. Isoconversional vs. model fitting methods: a case study of crystallization kinetics of a Fe-based metallic glass. J Therm Anal Calorim. 2007;89:399–405.
Burnham AK, Dinh LN. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J Therm Anal Calorim. 2007;89:479–90.
Janković B. Kinetic analysis of the nonisothermal decomposition of potassium metabisulfite using the model-fitting and isoconversional (model-free) methods. Chem Eng J. 2008;139:128–35.
Galwey AK. What is meant by the term ‘variable activation energy’ when applied in the kinetic analyses of solid state decompositions (crystolysis reactions)? Thermochim Acta. 2003;397:249–68.
Janković B, Adnaðević B, Jovanović J. Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: thermogravimetric analysis, Thermochim Acta. 2007;452:106–15.
Pourghahramani P, Forssberg E. Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods. Thermochim Acta. 2007;454:69–77.
Serra R, Nomen R, Sempere J. The non-parametric kinetics: a new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Serra R, Nomen R, Sempere J. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316:37–45.
Criado JM, Málek J, Ortega A. Applicability of the master plots in kinetic analysis of a non-isothermal rate. Thermochim Acta. 1989;147:377–85.
Tiptipakorn S, Damrongsakkul S, Ando S, Hemvichian K, Rimdusit S. Thermal degradation behaviors of polybenzoxazine and silicon-containing polyimide blends. Polym Degrad Stabil. 2007;92:1265–78.
Tang W, Liu Y, Zhang H, Wang C. New approximate formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal Calorim. 1977;11:445–9.
Flynn JH. The ‘temperature integral’: its use and abuse. Thermochim Acta. 1997;300:83–92.
Tesner PA. The activation energy of gas reactions with solid carbon. Eight International Symposium on Combustion, Williams & Wilkins Co., Baltimore, USA, 1962, pp. 807–13; Discussion by Essenhigh RH. pp. 813–14.
Acknowledgements
The authors express their sincere gratitude to the Director, Central Glass and Ceramic Research Institute (CG & CRI), India for his kind permission to publish this study. The authors are also grateful to the members of Analytical Facility Division and Materials Characterization Unit of CG & CRI, India for their extensive help in carrying out all the TEM analysis and TG experiments, respectively. The first author acknowledges the financial support of the Council of Scientific and Industrial Research (CSIR), India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, S., Das, P.K. Non-isothermal oxidation kinetics of single- and multi-walled carbon nanotubes up to 1273 K in ambient. J Therm Anal Calorim 107, 1093–1103 (2012). https://doi.org/10.1007/s10973-011-1797-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1797-x