Abstract
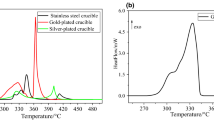
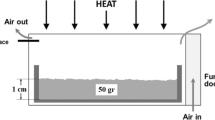
The thermal decomposition of potassium bromate (KBrO3) has been studied as a function of particle size, in the range 53–150 μm, by isothermal thermogravimetry at different temperatures, viz. 668, 673, 678, and 683 K in static air atmosphere. The theoretical and experimental mass loss data are in good agreement for the thermal decomposition of all samples of KBrO3 at all temperatures studied. The isothermal decomposition of all samples of KBrO3 was subjected to both model fitting and model-free (isoconversional) kinetic methods of analysis. Isothermal model fitting analysis shows that the thermal decomposition kinetics of all the samples of KBrO3 studied can be best described by the contracting square equation. Contrary to the expected increase in rate followed by a decrease with decrease in particle size, KBrO3 shows a regular increase in rate with reduction in particle size, which, we suggest, is an impact of melting of this solid during decomposition.








Similar content being viewed by others
Notes
Contracting square and contracting cube model equations: For crystals with instantaneous nucleation over all the surface, the nucleus growth takes place inwards to the centre of the crystal and the rate will be deceleratory throughout the process as the interface area will be decreasing progressively. The growth of the product inwards is considered to take place in different manners. In contracting cube model, the inward growth of the product will be considered in the form of a cube, while in contracting square model it is considered on the basis of decreasing interfacial contact area.
References
Section 172.730 Potassium bromate. Food additives permitted for direct addition to food for human consumption, US Code of Federal Regulations. US Food and Drug Administration.
Chipman JK, Davies JE, Parson JL, O’Neill G, Fawell JK. DNA oxidation by potassium bromate; a direct mechanism or link to lipid peroxidation? Toxicology. 1988;126:93–102.
Ueno H, Oishi K, Sayato Y, Nakamuro K. Oxidative cell damage in kat-sod assay of oxyhalides as inorganic disinfection by products and their occurrence by ozonation. Arch Environ Contam Toxicol. 2000;38:1–6.
IARC-Summaries & Evaluations: Potassium bromate (Group 2B), International Agency for Research on Cancer.
Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y. Toxicity and carcinogenicity of potassium bromate—a new renal carcinogen. Environ Health Perspect. 1990;87:309–35.
Galwey AK, Brown ME. Thermal decomposition of ionic solids. Amsterdam: Elsevier; 1999.
Stern KH. High temperature properties and thermal decomposition of inorganic salts with oxy anions. Florida: CRC Press LLC; 2001.
Vyazovkin S. Thermal analysis. Anal Chem. 2004;76:3299–312.
Deng C, Cai J, Liu R. Kinetic analysis of solid state reactions: evaluation of approximations to temperature integral and their applications. Solid State Sci. 2009;11:1375–9.
Vecchio S, Rodante F, Tomassetti M. Thermal stability of disodium and calcium phosphomycin and the effects of the excipients evaluated by thermal analysis. J Pharm Biomed Anal. 2001;24:1111–23.
Huang Y, Cheng Y, Alexander K, Dollimore D. The thermal analysis study of the drug captopril. Thermochim Acta. 2001;367:43–58.
Dollimore D, O’Connell C. A comparison of the thermal decomposition of preservatives, using thermogravimetry and rising temperature kinetics. Thermochim Acta. 1998;324:33–48.
Halikia I, Neou-Syngouna P, Kolitsa D. Isothermal kinetic analysis of the thermal decomposition of magnesium hydroxide using thermogravimetric data. Thermochim Acta. 1998;320:75–88.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Rodante F, Vecchio S, Tomassetti M. Kinetic analysis of thermal decomposition for penicillin sodium salts: model-fitting and model-free methods. J Pharm Biomed Anal. 2002;29:1031–43.
Brown ME. Introduction to thermal analysis: techniques and applications. 2nd ed. The Netherlands: Kluwer Academic Publishers; 2001.
Malek J, Mitsuhashi T, Criado M. Kinetic analysis of solid-state processes. J Mater Res. 2001;16:1862–71.
Zhou D, Schmitt EA, Zhang GG, Law D, Vyazovkin S, Wight CA, Grant DJW. Crystallization kinetics of amorphous nifedipine studied by model-fitting and model-free approaches. J Pharm Sci. 2003;92:1779–91.
Benderskii VA, Makarov DE, Wight CA. Chemical dynamics at low temperatures. New York: Wiley; 1994. p. 385.
Brown ME, Dollimore D, Galwey AK. Reactions in the solid state, comprehensive chemical kinetics, vol. 22. Amsterdam: Elsevier; 1980. p. 340.
Vyazovkin S, Wight CA. Isothermal and nonisothermal reaction kinetics in solids: in search of ways toward consensus. J Phys Chem. 1997;101A:8279–84.
Brill TB, James KJ. Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem Rev. 1993;93:2667–92.
Mark HF, Bikales NM, Overberger CG, Menges G, editors. Encyclopedia of polymer science and engineering. New York: Wiley; 1989. p. 231, 690.
Vyazovkin S, Wight CA. Isothermal and nonisothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17:407–33.
Dollimore D. Thermal analysis. Chem Rev. 1996;68:63–72.
Galwey AK. Is the science of thermal analysis kinetics based on solid foundations? A literature appraisal. Thermochim Acta. 2004;413:139–83.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Kotler JM, Hinman NW, Richardson CD, Scott JR. J Therm Anal Calorim. 2010;102:23–9.
Bertol CD, Cruz AP, Stulzer HK, Murakami FS, Silva MAS. Thermal decomposition behaviour of potassium and sodium jasorite synthesized in the presence of methyl amine and alanine. J Therm Anal Calorim. 2010;102:187–92.
Bancroft GM, Gesser HD. The search for perbromate. I: The thermal decomposition of bromates. J Inorg Nucl Chem. 1965;27:1545–56.
Jach J. Decomposition of metal perhalates, halates and halites. In: de Boer JH, editors. Reactivity of solids. Amsterdam: Elsevier; 1960. p. 334.
Jach J. The thermal decomposition of NaBrO3 part I—unirradiated material. J Phys Chem Solids. 1963;24:63–73.
Solymosi F. Structure and stability of salts of halogen oxyacids in the solid phase. London: John Wiley & Sons; 1977.
Breusov CN, Kashina NI, Revzina TV. Thermal decomposition of chlorates, bromates, iodates, perchlorates and periodates of potassium, rubidium and cesium. Zh Neorg Khim. 1970;15:612–24.
Prout EG, Tompkins FC. The thermal decomposition of potassium permanganate. Trans Faraday Soc. 1944;40:488–97.
Diefallah EM, Basahl SN, Obaid AY, Abu-Eittah RH. Kinetic analysis of thermal decomposition reactions: I. Thermal decomposition of potassium bromate. Thermochim Acta. 1987;111:49–56.
Joseph J, Nair TDR. Effect of metal oxide catalysts on thermal decomposition of potassium bromate. J Therm Anal Calorim. 1978;14:271–9.
Mohanty SR, Patnaik D. Effects of admixtures of potassium bromide on the thermal decomposition of potassium bromate. J Therm Anal Calorim. 1989;35:2153–9.
Das BC, Patnaik D. Effect of anion doping on the thermal decomposition of potassium bromate. J Therm Anal Calorim. 2000;61:879–83.
Kannan MP, Abdul Mujeeb VM. Effect of dopant ion on the kinetics of thermal decomposition of potassium bromate. React Kinet Catal Lett. 2001;72:245–52.
Abdul Mujeeb VM, Muraleedharan K, Kannan MP, Devi TG. Influence of trivalent ion dopants on the thermal decomposition kinetics of potassium bromate. Thermochim Acta (submitted).
Abdul Mujeeb VM, Aneesh MH, Muraleedharan K, Devi TG, Kannan MP. Effect of precompression on isothermal decomposition kinetics of potassium bromate. J Therm Anal Calorim. doi:10.1007/s10973-010-1127-8.
Kannan MP, Muraleedharan K. Kinetics of thermal decomposition of sulphate-doped potassium metaperiodate. Thermochim Acta. 1990;158:259–66.
Vyazovkin S, Wight CA. Kinetics in solids. Annu Rev Phys Chem. 1997;48:125–49.
Kenneth S, editor. Principles of solid-state chemistry. Reactions in solids. London: MacLaren & Sons Ltd.; 1968. p. 21.
Muraleedharan K, Kannan MP, Ganga Devi T. Thermal decomposition kinetics of potassium iodate. J Therm Anal Calorim. 2011;103:943–55.
Philips BR, Taylor O. Thermal decomposition of potassium metaperiodate. J Chem Soc 1963:5583–90.
Muraleedharan K, Kannan MP. Effects of dopants on the isothermal decomposition kinetics of potassium metaperiodate. Thermochim Acta. 2000;359:161–8.
Muraleedharan K, Kannan MP, Gangadevi T. Effect of metal oxide additives on the thermal decomposition kinetics of potassium metaperiodate. J Therm Anal Calorim. 2010;100:177–82.
Muraleedharan K, Kannan MP, Gangadevi T. Thermal decomposition of potassium metaperiodate doped with trivalent ions. Thermochim Acta. 2010;502:24–9.
Markovitz MM, Boryta DA. The decomposition kinetics of lithium perchlorate. J Phys Chem. 1961;65:1419–24.
Kim S, Kavitha D, Yu TU, Jung JS, Song JH, Lee SW, Kong SH. Using isothermal kinetic results to estimate kinetic triplet of pyrolysis reaction of polypropylene. J Anal Appl Pyro. 2008;81:100–5.
Nakamura H, Sakumoto K, Hara Y, Ochi K. Thermal analysis of sodium azide. J Hazard Mater. 1994;38:1–12.
Bircumshaw LL, Newman BH. The thermal decomposition of ammonium perchlorate II. The kinetics of the decomposition, the effect of particle size, and discussion of results. Proc Roy Soc Lond. 1955;A227:228–41.
Pai Verneker VR, Kishore K, Kannan MP. Effect of pretreatment on the sublimation of ammonium perchlorate. J Appl Chem Biotechnol. 1977;27:309–17.
Maycock JN, Pai Verneker VR, Rouch L Jr. Influence of growth parameters on the reactivity of ammonium perchlorate. Inorg Nucl Chem Lett. 1968;4:119–23.
Boldyrev VV, Avvakumov L. Mechanochemistry of inorganic solids. Russ Chem Rev. 1971;40:847–59.
Pai Verneker VR, Maycock JN. The thermal decomposition of ammonium perchlorate at low temperature. J Inorg Nucl Chem. 1967;29:2723–30.
Mitchell JW, DeVries RC, Roberts RW, Cynon P, editors. Reactivity of solids. New York: Wiley-Interscience; 1969. p. 287.
Muraleedharan K, Abdul Mujeeb VM, Aneesh MH, Ganga Devi T, Kannan MP. Effect of pre-treatments on isothermal decomposition kinetics of potassium metaperiodate. Thermochim Acta. 2010;510:160–7.
Huang Y, Risha GA, Yang V, Yetter RA. Effect of particle size on combustion of aluminum particle dust in air. Combust Flame. 2009;156:5–13.
Chou CJ, Olsen FA. Isothermal decomposition of isothiocyanatopentaamine cobalt(III) perchlorate. Particle size effect. Anal Chem. 1972;44:1841–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdul Mujeeb, V.M., Muraleedharan, K., Kannan, M.P. et al. The effect of particle size on the thermal decomposition kinetics of potassium bromate. J Therm Anal Calorim 108, 1171–1182 (2012). https://doi.org/10.1007/s10973-011-1733-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1733-0