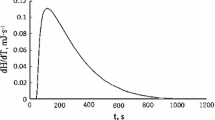
Abstract
Laburnine’s dissolution behaviors in glucose and saline solution were studied by a micro-calorimetry method. The measured integral and differential heats of solution were utilized to build equations of the solute and the heat, so that dissolution thermodynamic equations and half-time periods, Δsol H m, Δsol G m, and Δsol S m were obtained. The results show that this study does not only provide a simple method for the determination of the half-life period for a drug but also offer a theoretical reference for the clinical application of laburnine.




Similar content being viewed by others
References
Amroyan EA. Participation of type “E” prostaglandins in the effects of cytosine on cerebral vessel resistance. Eksp Klin Med. 1977;17:3–7.
Wei QH, Zhao BG. Alkaloids of Thermopsis lanceolata R. Brown and their biological activities. J Nanjing For Univ (Nat Sci Ed). 2000;24:73–5 (in Chinese).
Wang JX, Wang GJ. Progress on pharmacology and clinical application of matrine and oxymatrine. Chin Hepatol. 2000;5:116–7.
Sun XJ, Xu XY, Liu M, Li LW, Sun DZ. Enthalpic interactions of anti-tumor drug matrine in aqueous sodium chloride solutions. J Therm Anal Calorim. 2010;100:1073–7.
Li ZX, Pu XH, Zhao WW. Dissolution properties of oxymatrine in citric acid solution. J Therm Anal Calorim. 2011;104:1191–4.
Montgomery RL, Melaugh RA, Lau CC, Meier GH, Chan HH, Rossini FD. Determination of the energy equivalent of a water solution calorimeter with a standard substance. J Chem Thermodyn. 1977;9:915–36.
Xue L, Zhao FQ, Xing XL. Dissolution properties of 1,3,3-trinitroazetidine in ethyl acetate and N,N-dimethylformamide. Acta Phys Chim Sin. 2009;25:2413–6.
Gao SL, Chen SP, Hu RZ, Li HY, Shi QZ. Derivation and application of theromodynamic equations. Chin J Inorg Chem. 2002;18:362–6 (in Chinese).
Acknowledgements
This study was supported by the Phytochemistry Key Laboratory of Shaanxi Province (No. 09JS066), and the Project Foundation of Shaanxi Province (No. 2006k16-G16) in the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z.X., Pu, X.H. & Zhao, W.W. Thermodynamic properties of laburnine in saline and glucose solutions. J Therm Anal Calorim 107, 1339–1344 (2012). https://doi.org/10.1007/s10973-011-1723-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1723-2