Abstract
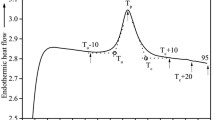
Starches with varying amylose content, one hydroxypropylated high amylose starch and two thermoplastic starches (pre-gelatinized and with V type crystals) were gelatinized in the presence of added water. Gelatinization was characterized using wide-angle X-ray scattering and modulated temperature differential scanning calorimetry (mT-DSC) with a heat-cool temperature profile. The gelatinization endotherms were recorded in total heat capacity curves that were resolved into storage (reversing) and loss heat capacities, and non-reversing heat capacity curve. The endotherms were mainly of non-reversing nature, with a small contribution from the reversing component. Starch melting is a plasticizer-assisted disruption of crystals and other structures such as starch–lipid complexes and granules. Reversibility was limited since the native amylopectin crystals are rarely recrystallized and starch–lipid complexes do not reform. Recrystallization is predominantly due to subsequent slow formation of V type amylose crystals, with some B type due to recrystallization of amylopectin.






Similar content being viewed by others
References
Dos Santos Rosa D, Bardi MAG, Machado LDB, Dias DB, Silva LGA, Kodama Y. Influence of thermoplastic starch plasticized with biodiesel glycerol on thermal properties of PP blends. J Therm Anal Calorim. 2010;99:675–9.
Schlemmer D, Sales MJA. Thermoplastic starch films with vegetable oils of Brazilian Cerrado thermal characterization. J Therm Anal Calorim. 2009;93:599–604.
Rudnik E. Thermal properties of biocomposites. J Therm Anal Calorim. 2009;88:495–8.
Gill PS, Sauerbrunn SR, Reading M. Modulated differential scanning calorimetry. J Therm Anal Calorim. 1993;40:931–9.
Zobel HF. Starch crystal transformations and their industrial importance. Starch. 1988;40:1–7.
Cura JA, Jansson PE, Krisman CR. Amylose is not strictly linear. Starch. 1995;47:207–9.
Manners DJ. Recent developments in our understanding of amylopectin structure. Carbohydr Polym. 1989;11:87–112.
Le Bail P, Bizot H, Pontoire B, Buleon A. Polymorphic transitions of amylose–ethanol crystalline complexes induced by moisture exchanges. Starch. 1995;47:229–32.
Le Bail P, Bizot H, Buleon A. ‘B’ to ‘A’ type phase transition in short amylose chains. Carbohydr Polym. 1993;21:99–104.
Godet MC, Buleon A, Tran V, Colonna P. Structural features of fatty acid–amylose complexes. Carbohydr Polym. 1993;21:91–5.
Wu HC, Sarko A. The double-helical molecular structure of crystalline b-amylose. Carbohydr Res. 1978;61:7–25.
Imberty A, Chanzy H, Pe’rez S, Buleon A, Tran V. The double-helical nature of the crystalline part of A-starch. J Mol Biol. 1988;201:365–78.
Imberty A, Perez S. A revisit to the three-dimensional structure of B-type starch. Biopolymers. 1988;27:1205–21.
Imberty A, Buléon A, Tran V, Pérez S. Recent advances in knowledge of starch structure. Starch. 1991;43:375–84.
Cheetham NWH, Tao L. Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydr Polym. 1998;36:277–84.
Perry PA, Donald AM. The role of plasticization in starch granule assembly. Biomacromolecules. 2000;1:424–32.
Mani R, Bhattacharya M. Properties of injection moulded blends of starch and modified biodegradable polyesters. Eur Polym J. 2001;37:515–26.
Ma XF, Yu JG. Formamide as the plasticizer for thermoplastic starch. J Appl Polym Sci. 2004;93:1769–73.
Pavlovic S, Brandao PRG. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Minerals Eng. 2003;16:1117–22.
Sang Y, Alavi S, Shi YC. Subzero glass transition of waxy maize starch studied by differential scanning calorimetry. Starch. 2009;61:687–95.
Liu Y, Shi YC. Phase and state transitions in granular starches studied by dynamic differential scanning calorimetry. Starch. 2006;58:433–42.
Liu H, Xie F, Yu L, Chen L, Li L. Thermal processing of starch-based polymers. Prog Polym Sci. 2009;34:1348–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanks, R.A., Gunaratne, L.M.W.K. Gelatinization and retrogradation of thermoplastic starch characterized using modulated temperature differential scanning calorimetry. J Therm Anal Calorim 106, 93–99 (2011). https://doi.org/10.1007/s10973-011-1620-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1620-8