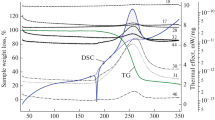
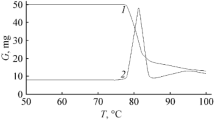
Abstract
New energetic compounds-3,4,5-1H-trinitropyrazole (TNP), 1-methyl-3,4,5-1H-trinitropyrazole (MTNP) and ammonium 3,4,5-1H-TNP have been synthesized and characterized by thermal analysis. These new compounds can be considered as promising since the high heat of formation for them. To estimate the process of their thermal decomposition, the original technique for computer simulation was used. We generated the models for the mechanisms of thermal decay of synthesized compounds which allowed obtaining comprehensive spectrum of transformations of intermediates on the way to the final products of thermolysis. The preferred pathways were determined based on the results of activation energy (E a) calculations (DFT 6-311++G** method) of thermal decay reactions for each generated pathways. The thermal decomposition has been studied also experimentally by thermogravimetry (TG) and differential scanning calorimetry. Kinetic parameters of thermolysis were evaluated by model-free and -fitting methods using TG data. Model-free method has given not reliable data for TNP and MTNP compounds, whereas model-fitting yields kinetic equations with the good correlation with experimental TG data.










Similar content being viewed by others
References
Dalinger IL, Popova GP, Vatsadze IA, Shkineva TK, Shevelev SA. Synthesis of 3, 4, 5-trinitropyrazole. Russ Chem Bull Int Ed. 2009;58:2185.
Dalinger IL, Vatsadze IA, Popova GP, Shkineva TK, Shevelev SA. The specific reactivity of 3,4,5-trinitro-1H-pyrazole. Mendeleev Commun. 2010;20:253–4.
Herve G, Roussel C, Graindorge H. Selective preparation of 3,4,5-trinitro-1H-pyrazole: a stable all-carbone-nitrated arene. Angewandte Chem. 2010;49:3177–81.
Korolev V, Pivina T, Porollo A, Petukhova T, Sheremetev A, Ivshin V. Differentiation of the molecular structures of nitro compounds as the basis for simulation of their thermal destruction processes. Russian Chem Rev. 2009;78(10):945–69.
Koch W, Holthausen MC. A chemist’s guide to density functional theory. Weinheim: Wiley-VCH; 2001. 300 pp.
Clark T. A handbook of computational chemistry. New York: Wiley; 1985. 383 pp.
Frish MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Jr., Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komazomi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzales C, Head-Gordon M, Replogle ES, and Pople JA, GAUSSIAN 98. Revision A.9, Gaussian Inc., Pittsburgh, 1998.
Emanuel NM, Knorre DG. Course of chemical kinetics (in Russian). Moscow: Vysshaya Shkola; 1984. 463 pp.
Manelis GB, Nazin GM, YuI Rubtsov, Strunin VA. Termicheskoe razlozhenie i gorenie vzryvchatykh veshchestv i porokhov (in Russian). Moscow: Nauka; 1996. 223 pp.
Khrapkovskii GM, Marchenko GN, Shamov AG. Influence of structure of molecules on kinetic parameters of monomolecular decay of C- and N-nitro compounds (in Russian). Kazan: FEN; 1997. 139 pp.
Korolev VL, Petukhova TV, Pivina TS, Porollo AA, Sheremetev AB, Suponitsky KY, Ivshin VP. Thermal decomposition mechanisms of nitro-1,2,4-triazols: a theoretical study. Russian Chem Bull. 2006;55(8):1338–410.
Jank H-W, Meister A. Zerlegung von Spektren in ihre Komponenten. I. Mathematische Probleme. Kulturpflanze. 1982;30:125−40.
Acknowledgements
We thank Professor Boris Korsunskii (Semenov Institute of Chemical Physics, Russian Academy of Science) for the helpful discussions of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalinger, I., Shevelev, S., Korolev, V. et al. Chemistry and thermal decomposition of trinitropyrazoles. J Therm Anal Calorim 105, 509–516 (2011). https://doi.org/10.1007/s10973-010-1213-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1213-y