Abstract
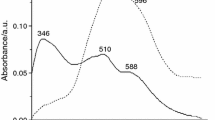
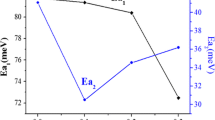
In the present work the LaCoO3 formation from gel precursors obtained by water-based sol–gel method with citric acid was studied. As precursors La and Co nitrates were used. The obtained gels were analyzed by TG/DTA and TG/AGE. The decomposition of the gels takes place in two main steps with the evolution of the same volatile compounds (H2O, CO2 si NO2) leading to the conclusion that two types of bonding of the components in the gels occurred. The decomposition of the gels takes place up to 400 °C. The gels thermally treated at 600 °C lead to single pure perovskite rhombohedral phase of lanthanum cobalt oxide (LaCoO3).









Similar content being viewed by others
References
Yamazoe N, Teraoka Y. Oxidation catalysis of perovskites—relationships to bulk structure and composition (valency, defect, etc.). Catal Today. 1990;8:175–99.
Pena MA, Fierro JLG. Chemical structures and performance of perovskite oxides. Chem Rev. 2001;101:1981–2017.
Arai H, Yamada T, Eguchi K, Seiyama T. Calaytic combustion of methanen over various perovskite type oxide. Appl Catal. 1986;26:265–76.
Svegl F, Orel B. Preparation of lithiated Co- and Ni-oxide powders and thin films by wet chemistry processing. J Sol–Gel Sci Technol. 1999;14:187–201.
Delmon B. Preparation of heterogeneous catalysts: synthesis of highly dispersed solids and their reactivity. J Therm Anal Calorim. 2007;90(1):49–65.
Sinquin G, Petit C, Hindermann JP, Kiennemann A. Study of the formation of LaMO3 (M=Co, Mn) perovskites by propionates precursors: application to the catalytic destruction of chlorinated VOCs. Catal Today. 2001;70:183–96.
Nakayama S, Okazaki M, Aung YL, Sakamoto M. Preparations of perovskite-type oxides LaCoO3 from three different methods and their evaluation by homogeneity, sinterability and conductivity. Solid State Ionics. 2003;158:133–9.
Rane KS, Uskaikar H, Pednekar R, Mhalsikar R. The low temperature synthesis of metal oxides by novel hydrazine method. J Therm Anal Calorim. 2007;90(3):627–38.
Kakihana M. Sol-gel preparation of high temperature superconducting oxides. J Sol–Gel Sci Technol. 1996;6:7–55.
Predoanã L, Malic B, Kosec M, Carata M, Caldararu M. Characterization of LaCoO2 powders obtained by water based sol-gel method with citric acid. J Eur Ceram Soc. 2007;27:4407–11.
Livage J. Sol-gel synthesis of heterogeneous catalysts from aqueous solutions. Catal Today. 1998;4:3–19.
Tsai MT. Effects of hydrolysis processing of the characterization of forsterite gel fibers. Part I. Preparation, spinability and molecular structure. J Eur Ceram Soc. 2002;22:3–19.
Livage J, Henry M, Sanchez C. Sol-gel chemistry of transition metal oxides. J Non-Cryst Solids. 1988;18:259–341.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Predoana, L., Malic, B. & Zaharescu, M. LaCoO3 formation from precursors obtained by water-based sol–gel method with citric acid. J Therm Anal Calorim 98, 361–366 (2009). https://doi.org/10.1007/s10973-009-0315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0315-x