Abstract
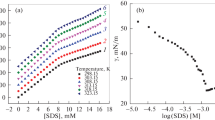
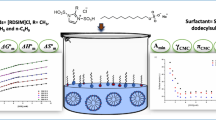
The micellization characteristics of sodium n-dodecyl sulfate (SDS) have been investigated by microcalorimetric technique at conditions close to the physiological ones. The thermodynamics of micellization were studied at 20, 25, 30, 35 and 40 °C in 50 mM HEPES buffer, pH 7.4 and 160 mM NaCl using isothermal titration calorimetric (ITC) technique. The calorimeter can operate in a stepwise addition mode, providing an excellent method of determination of critical micelle concentration (CMC) and enthalpy of demicellization (and hence micellization). It can as well distinguish between aggregating and non-aggregating amphiphiles (solutes) in solution. The dilution enthalpy (∆H dil) was calculated and graphed versus concentration in order to determine the micellization enthalpy (∆H mic) and CMC. In addition to the CMC and ∆H mic, the effective micellar charge fraction (α) of the ionic surfactant micellization process can also be determined from ITC curves. The Gibbs free energy of the micellization (∆G mic), entropy of the micellization (∆S mic), and specific heat capacity of the micellization (∆C P,mic) process have been evaluated by the direct calorimetric method (mass-action model) as well as by the indirect method of van’t Hoff by processing the CMC and α results of microcalorimetry at different temperatures. The differences of the results obtained by these two procedures have been discussed. The presence of NaCl (160 mM) in the solutions decreased the CMC of SDS. The enthalpy changes associated with micelle dissociation were temperature-dependent, indicating the importance of hydrophobic interactions. The ∆G mic was found to be negative, implying, as expected, that micellization occurs spontaneously once the CMC has been reached. The values of ∆G mic were found to become more negative with increasing temperature and the ∆S mic was found to decrease with increasing temperature in both models.




Similar content being viewed by others
References
Clint JH. Surfactant aggregation. New York: Chapman and Hall; 1991.
Moulik SP. Micelles: self-organized surfactants assemblies. Curr Sci. 1996;71:368–76.
Emerson MF, Holtzer A. Hydrophobic bond in micellar systems: effects of various additives on the stability of micelles of sodium dodecyl sulfate and of n-dodecyltrimethyl ammonium bromide. J Phys Chem. 1967;71:3320–30.
Tanford C. The hydrophobic effect: formation of micelles and biological membranes. New York: Wiley; 1980.
Moroi A. Micelles: theoretical and applied aspects. New York: Plenum Press; 1992.
Kumar S, David SL, Din K. Effects of various hydrocarbons on micellar growth. J Am Oil Chem Soc. 1997;74:797–801.
Sugioka H, Matsuoka K, Moroi Y. Temperature effect on formation of sodium cholate micelles. J Colloid Interface Sci. 2003;259:156–62.
Esposito C, Colicchio P, Facchiano A, Ragone R. Effect of a weak electrolyte on the critical micellar concentration of sodium dodecyl sulfate. J Colloid Interface Sci. 1998;200:310–2.
Ghosh S. Surface chemical and micellar properties of binary and ternary surfactant mixtures (cetylpyridinium chloride, tween-40, and brij-56) in an aqueous medium. J Colloid Interface Sci. 2001;244:128–38.
Ghosh S, Moulik SP. Interfacial and micellization behaviors of binary and ternary mixtures of amphiphiles (tween-20, brij-35, and sodium dodecyl sulfate) in aqueous medium. J Colloid Interface Sci. 1998;208:357–66.
McBain JW. Mobility of highly-charged micelles. Trans Faraday Soc. 1913;9:99–101.
Kresheck GC, Hargraves WA. Thermometric titration studies of the effect of head group, chain length, solvent, and temperature on the thermodynamics of micelle formation. J Colloid Interface Sci. 1974;48:481–93.
Mukherjee K, Mukherjee DC, Moulik SP. Thermodynamics of micellization of aerosol OT in binary mixtures of water, formamide, ethylene glycol, and dioxane. J Phys Chem. 1994;98:4713–8.
Jana PK, Moulik SP. Interaction of bile salts with hexadecyltrimethyl ammonium bromide and sodium dodecyl sulfate. J Phys Chem. 1991;95:9525–32.
Haque MdE, Das AR, Moulik SP. Behaviors of sodium deoxycholate (NaDC) and polyoxyethylene tert-octylphenyl ether (triton x-100) at the air/water interface and in the bulk. J Phys Chem. 1995;99:14032–8.
Bordbar AK, Hosseinzadeh R, Norozi MH. Interaction of a homologous series of n-alkyl trimethyl ammonium bromides with eggwhite lysozyme. J Therm Anal Cal. 2007;87:453–6.
Majhi PR, Moulik SP. Energetics of micellization: reassessment by a high-sensitivity titration microcalorimeter. Langmuir. 1998;14:3986–90.
Chatterjee A, Maiti S, Sanyal SK, Moulik SP. Micellization and related behaviors of n-cetyl-n-ethanolyl-n,n-dimethyl and n-cetyl-n,n-diethanolyl-n-methyl ammonium bromide. Langmuir. 2002;18:2998–3004.
Razafindralambo H, Dufour S, Paquot M, Deleu M. Thermodynamic studies of the binding interactions of surfactin analogues to lipid vesicles. J Therm Anal Cal. 2009;95:817–21.
Mazer NA, Olofsson G. Calorimetric studies of micelle formation and micellar growth in sodium dodecyl sulfate solutions. J Phys Chem. 1982;86:4584–93.
Gu G, Yan H, Chen W, Wang W. Observation of micelle formation and micellar structural transition from sphere to rod by microcalorimetry. J Colloid Interface Sci. 1996;178:614–9.
Chen D, Zhu JX, Yuan P, Yang SJ, Chen T-H, He HP. Preparation and characterization of anion-cation surfactants modified montmorillonite. J Therm Anal Cal. 2008;94:841–8.
Leharne SA. Calorimetric analysis of vesicular systems. J Therm Anal Cal. 2002;69:465–85.
Lagergel S, Kamyshny A, Magdassi S, Partyka S. Calorimetric methods applied to the investigation of divided systems in colloid science. J Therm Anal Cal. 2003;71:291–310.
Dai S, Tam KC. Isothermal titration calorimetric studies on the temperature dependence of binding interactions between poly(propylene glycol)s and sodium dodecyl sulfate. Langmuir. 2004;20:2177–83.
Stodghill SP, Smith AE, O’Haver JH. Thermodynamics of micellization and adsorption of three alkyltrimethyl ammonium bromides using isothermal titration calorimetry. Langmuir. 2004;20:11387–92.
Thongngam M, McClements DJ. Influence of pH, ionic strength, and temperature on self-association and interactions of sodium dodecyl sulfate in the absence and presence of chitosan. Langmuir. 2005;21:79–86.
Paula S, Sus W, Tuchtenhagen J, Blume A. Thermodynamics of micelle formation as a function of temperature: a high sensitivity titration calorimetry study. J Phys Chem. 1995;99:11742–51.
Johnson I, Olofsson G, Jonsson B. Micelle formation of ionic amphiphiles. Thermochemical test of a thermodyanamic model. J Chem Soc Faraday Trans. 1987;83:3331–44.
Bergstrom S, Olofsson G. A calorimetric study of three long-chain ionic surfactants. Thermochim Acta. 1986;109:155–64.
Bandopadhyay A, Moulik SP. Counterion binding behavior of micelles of sodium dodecyl sulphate and bile salts in the pure state, in mutually mixed states and mixed with a nonionic surfactant. Colloid Polym Sci. 1988;266:455–61.
Blandamer MJ, Cullis PM, Pure JBFN. Calorimetric studies of macromolecular aqueous solutions. Pure Appl Chem. 1996;68:1577–82.
Bijma K, Engberts J, Blandamer MJ, Cullis PM, Last PM, Irlam KD, et al. Classification of calorimetric titration plots for alkyltrimethyl ammonium and alkylpyridinium cationic surfactants in aqueous solutions. J Chem Soc Faraday Trans. 1997;93:1579–84.
Stenius P, Backlund S, Ekwall P. Thermodynamic and transport properties of organic salts. IUPAC Chem Data Ser. 1980;28:295.
Hait SK, Moulik SP, Palepu R. Refined method of assessment of parameters of micellization of surfactants and percolation of w/o microemulsions. Langmuir. 2002;18:2471–6.
Dai S, Tam KC. Isothermal titration calorimetric studies of alkyl phenol ethoxylate surfactants in aqueous solutions. Colloids Surf A: Physicochem Eng Aspects. 2003;229:157–68.
McClements DJ. Isothermal titration calorimetry study of pectin-ionic surfactant interactions. J Agric Food Chem. 2000;48:5604–11.
Olofsson G, Wang G. Polymer-surfactant systems: isothermal titration and temperature scanning calorimetric studies of polymer-surfactant systems. New York: Marcel Dekker; 1998.
Ananthapadmanabhan KP. Interactions of surfactants with polymers and proteins: surfactants solutions, adsorption and aggregation properties. London: CRC Press; 1993.
Thongngam M, McClements DJ. Characterization of interactions between chitosan and an anionic surfactant. J Agric Food Chem. 2004;52:987–91.
Wangsakarn A, Chinachoti P, McClements DJ. Maltodextrin-anionic surfactant interactions: isothermal titration calorimetry and surface tension study. J Agric Food Chem. 2001;49:5039–45.
Jonsson B, Lindman B, Holmberg K, Kronberg B. Surfactant and polymers in aqueous solution. New York: Wiley; 1998.
Fuguet E, Rafols C, Bosch E, Roses M. Characterization of the solvation properties of sodium n-dodecyl sulfate micelles in buffered and unbuffered aqueous phases by solvatochromic indicators. Langmuir. 2003;19:55–62.
Mukerjee P. The nature of the association equilibria and hydrophobic bonding in aqueous solutions of association colloids. Adv Colloid Interface Sci. 1967;1:242–75.
Attwood D, Florence AT. Surfactant systems: their chemistry, pharmacy and biology. New York: Chapman and Hall; 1983.
Wang Y, Han B, Yan H, Kwak JCT. Microcalorimetry study of interaction between ionic surfactants and hydrophobically modified polymers in aqueous solutions. Langmuir. 1997;13:3119–23.
Chatterjee A, Moulik SP, Sanyal SK, Mishra BK, Puri PM. Thermodynamics of micelle formation of ionic surfactants: a critical assessment for sodium dodecyl sulfate, cetylpyridinium chloride and dioctyl sulfosuccinate (Na salt) by microcalorimetric, conductometric, and tensiometric measurements. J Phys Chem B. 2001;105:12823–31.
Rosen MJ. Surfactants and interfacial phenomena. New York: Wiley; 1989.
Evans DF, Wennerstrom H. The colloidal domain where physics, chemistry, biology and technology meet. New York: Wiley; 1999.
Kresheck GC. Comparison of the calorimetric and van’t Hoff enthalpy of micelle formation for a nonionic surfactant in H2O and D2O solutions from 15 to 40 °C. J Phys Chem B. 1998;102:6596–600.
Franks F, Reid DS. Water: a comprehensive treatise. New York: Plenum Press; 1975.
Naghibi H, Tamura A, Sturtevant JM. Significant discrepancies between van’t Hoff and calorimetric enthalpies. Proc Natl Acad Sci USA. 1995;92:5597–99.
Onori G, Santucci A. Calorimetric study of the micellization of n-butoxyethanol in water. J Phys Chem B. 1997;101:4662–6.
Blume A, Tuchtenhagen J, Paula S. Application of titration calorimetry to study binding of ions, detergents, and polypeptides to lipid bilayers. Prog Colloid Polym Sci. 1993;93:118–22.
Goddard ED, Benson GC. Conductivity of aqueous solutions of some paraffin chain salts. Can J Chem. 1957;35:986–91.
Acknowledgements
The financial supports of this work by the Research Council of Isfahan University are gratefully acknowledged (grant number 861129).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taheri-Kafrani, A., Bordbar, AK. Energitics of micellizaion of sodium n-dodecyl sulfate at physiological conditions using isothermal titration calorimetry. J Therm Anal Calorim 98, 567–575 (2009). https://doi.org/10.1007/s10973-009-0170-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0170-9