Abstract
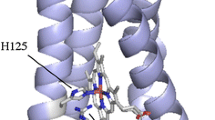
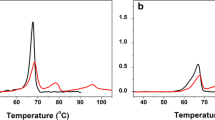
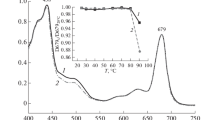
We attempted to determine the experimental conditions under which poplar plastocyanin iso-forms PCa and PCb undergo reversible thermal unfolding studied by differential scanning calorimetry (DSC). Our results indicate that an exothermic unfolding process exists always in the presence of molecular oxygen. Reversible unfolding and almost perfect two-state transitions were exhibited in the presence of TCEP under anaerobic conditions. This suggests that the second endothermic peak is due to copper-site disulfide dimmers formed during thermal denaturation. The conformational thermal stability of reduced PCb (ΔG (25 °C) = 33.9 kJ mol−1) has proven to be higher than that of reduced PCa (ΔG(25 °C) = 22.9 kJ mol−1).






Similar content being viewed by others
References
Privalov P, Potekhin S. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51.
Shosheva A, Donchev A, Dimitrov M, Kostov G, Toromanov G, Getov V, et al. Comperative study of the stability of poplar plastocyanin isoforms. Biochim Biophys Acta. 2005;1748(1):116–27.
Sanchez-Ruiz JM, Lopez-Lacomba JL, Cortijo M, Mateo PL. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988;27(5):1648–52.
Sanchez-Ruiz JM. Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry. Biophys J. 1992;61(4):921–35.
Milardi D, La Rosa C, Grasso D. Extended theoretical analysis of irreversible thermal protein unfolding. Biophys Chem. 1994;52(3):183–9.
Pappalardo M, Sciacca MFM, Milardi D, Grasso DM, La Rosa C. Thermodynamics of azurin folding. The role of copper ion. J Therm Anal Calorim. 2008;93(2):575–81.
Sykes A. Structure and electron-transfer reactivity of the blue copper protein plastocyanin. Chem Soc Rev. 1985;14:283–315.
Onodera J, Sugimura Y, Yoshizaki F. Novel plastocyanin containing phenyl-alanine-83 from the gymnosperm Ginkgo biloba. Protein Pept Lett. 2006;13(1):15–9.
Dimitrov M, Donchev A, Egorov C, Atanasov B. Complete amino acid sequence of poplar plastocyanin. FEBS Lett. 1987;226:17–22.
Dimitrov M, Donchev A, Egorov T. Microheterogeneity of Parsley plastocyanin. FEBS Lett. 1990;265:141–5.
Dimitrov M, Donchev A, Egorov TA. Twin plastocyanin dimorphism in Tobacco. Biochim Biophys Acta. 1993;1203(2):184–90.
Burkey K, Gizelice Z, Carter T. Genetic variation in soybean phosphosynthetic electron transport capacity is related to plastocyanin concentration in the chloroplast. Photosynth Res. 1996;49:1411–90.
Pesaresi P, Scharfenberg M, Weigel M, Granlund I, Schröder WP, Finazzi G, et al. Mutants, overexpressors, and interactors of Arabidopsis plastocyanin isoforms: revised roles of plastocyanin in photosynthetic electron flow and thylakoid redox state. Mol Plant 2009;2(2):236–48.
Salah EA-G. Contribution of plastocyanin isoforms to photosynthesis and copper homeostasis in Arabidopsis thaliana grown at different copper regimes. Planta. 2009;229:767–79.
Weigel M, Varotto C, Pesaresi P, Finazzi G, Rappoport F, Salmini F, et al. Plastocyanin is indispensable for photosynthetic electron flow in Arabidopsis thaliana. J Biol Chem. 2003;278(33):31286–9.
Shosheva A, Donchev A, Dimitrov M, Zlatanov I, Toromanov G, Getov V, et al. Experimental and numerical study of the polar plastocyanin isoforms using Tyr as a probe for electrostatic similarity and dissimilarity. Biochim Biophys Acta. 2004;1698(1):67–75.
Dobrikova AG, Dimitrov MI, Taneva SG, Petkanchin IB. Protein-coated β-ferric hydrous oxide particles: an electrokinetic and electrooptic study. Colloid Surf B: Biointerface. 2007;56(1–2):114–20.
Guzzi RC, Andolfi L, Cannistraro S, Verbeet MP, Canters GW, Sporelli L. Thermal stability of wild type and disulfide dridge containing mutant of poplar plastocyanin. Biophys Chem. 2004;112(1):35–43.
Sanberg A, Haroson D, Göran Karlsson B. Thermal denaturation of spinach plastocyanin: effect of copper site oxidation state and molecular oxygen. Biochemistry. 2003;42(34):10301–10.
Guzzi R, Sportelli L, La Rosa C, Milardi D, Grasso D, Verbeet MPh, et al. A spectroscopic and calorimetric investigation on the thermal stability of the Cys3Ala/Cys26Ala azurin mutant. Biophys J. 1999;77(2):1052–63.
Sanberg A, Leckner J, Ying S, Schwarz F, Göran Karlsson B. Effects of metal ligation and oxygen on the reversibility of the thermal denaturation of Pseudomonas aeruginosa azurin. Biochemistry. 2002;41(3):1060–9.
Feio M, Navarro J, Teixeira M, Harrison D, Göran Karlsson B, De la Rosa M. A thermal unfolding study of plastocyanin from the thermophilic cyanobacterium Phormidium laminosum. Biochemistry. 2004;43(46):14784–91.
Gross E, Draheim J, Curtiss A, Crombie B, Scheffer A, Pan B, et al. Thermal denaturation of plastocyanin: the effect of oxidation state, reductants and anaerobicity. Arch Biochem Biophys. 1992;298:413–19.
Privalov PL. Small globular proteins. Adv Protein Chem. 1979;33:167–241.
Privalov PL, Khechinashvili NN. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974;86(3):665–84.
Privalov PL. Thermodynamic bases of the stability of protein structure. Thermochim Acta. 1990;163:33–46.
Pace NC, Grimsley GR, Thomas ST, Makhatadze GI. Heat capacity change for ribonuclease A folding. Protein Sci. 1999;8(7):1500–4.
Loladze VV, Ermolenko DN, Makhatadze GI. Heat capacity changes upon burial of polar and nonpolar groups in proteins. Protein Sci. 2001;10(7):1343–52.
Murphy KP, Gill SJ. Solid model compounds and the thermodynamics of protein unfolding. J Mol Biol. 1991;22(3):2699–709.
Murphy KP, Freire E. Thermodynamics of structural stability and cooperative folding behavior in proteins. Adv Protein Chem. 1992;43:313–61.
Makhatadze GI, Privalov PL. Energetics of protein structure. Adv Protein Chem. 1995;47:307–425.
Makhatadze GI, Lopez MM, Richardson JM III, Thomas ST. Anion binding to the ubiquitin molecule. Protein Sci. 1998;7(3):689–97.
Spolar RS, Livingstone JR, Record MT Jr. Use of liquid hydrocarbon and amide transfer data to estimate contributions to thermodynamic functions of protein folding from the removal of nonpolar and polar surface from water. Biochemistry. 1992;31(16):3947–55.
Milardi D, La Rosa C, Grasso D, Guzzi R, Sporelli L, Fini C. Thermodynamics and kinetics of the thermal unfolding of plastocyanin. Eur Biophys J. 1998;27(3):273–82.
Kachalova GS, Bourenkov GP, Bartunik HD, Dimitrov MI, Donchev AA, Shosheva ACh. Crystal structure of poplar plastocyanin b. Acta Crystallogr. 2002;A58(Supplement):C303.
Sturtevant J. Biochemical applications of differential scanning calorimetry. Ann Rev Phys Chem. 1987;38:463–512.
Jelesarov I, Bosshard HR. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit. 1999;12(1):3–18.
Acknowledgements
This work was supported by grants of Fund “Scientific researches” of the Bulgarian Ministry of Education and Sciences (contract No. MY-Б-1511/2005 and No. Б-1519/05/2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Getov, V.I., Toromanov, G.R., Kostov, G.K. et al. Reversible unfolding of poplar iso-plastocyanins. J Therm Anal Calorim 98, 877–883 (2009). https://doi.org/10.1007/s10973-009-0141-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0141-1