Abstract
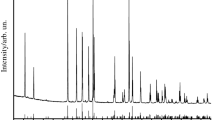
In the present work temperature dependence of heat capacity of rubidium niobium tungsten oxide has been measured first in the range from 7 to 395 K and then between 390 and 650 K, respectively, by precision adiabatic vacuum and dynamic calorimetry. The experimental data were used to calculate standard thermodynamic functions, namely the heat capacity \( C_{\text{p}}^{\text{o}} (T), \) enthalpy \( H^{\text{o}} ({\rm T}) - H^{\text{o}} (0) \), entropy \( S^{\text{o}} (T) - S^{\text{o}} \left( 0 \right) \), and Gibbs function \( G^{{^{\text{o}} }} ({\rm T}) - H^{{^{\text{o}} }} (0) \), for the range from T→0 to 650 K. The high-temperature X-ray diffraction and the differential scanning calorimetry were used for the determination of temperature and decomposition products of RbNbWO6.


Similar content being viewed by others
References
Yamamura H, Nishino H, Kakinuma K, Nomura K. Electrical conductivity anomaly around fluorite–pyrochlore phase boundary. Solid State Ionics. 2003;158:359–65.
Chamberlain SL, Hess ST, Corruccini LR. Dipolar magnetic order in the pyrochlore structure. Phys Lett A. 2004;323:310–4.
Hashizume T, Yokota A, Saiki A, Terayama K. Fabrication of potassium tantalate films by hydrothermal electrochemical method at low temperature. J Therm Anal Calorim. 2008;92:431–4.
Yudintsev SV. Corrosion study of actinide waste forms with Garnet-type structure. Geol Ore Deposit. 2003;45:151–7.
Chernorukov N, Knyazev A, Kuznetsova N, Markin A, Smirnova N. Crystal structure and thermodynamic properties of cesium tantalum tungsten oxide. Thermochim Acta. 2008;470:47–51.
Bydanov NN, Chernaya TS, Muradyan LA, Sarin VA, Rider EE, Yankovskii VK, et al. Neutron-diffraction refinement of atomic structures of crystals of RbNbWO6 and TlNbWO6. Kristallografiya. 1987;32:623–30.
Babel D, Pausewang C, Viebahn W. Die Struktur einiger fluoride, oxide und oxidfluoride AMe2X6, der RbNiCrF6 – Typ. Zeitschrift fuer Naturforschung, Teil B 1967;22:1219–20.
Varushchenko RM, Druzhinina AI, Sorkin EL. Low-temperature heat capacity of 1-bromoperfluorooctane. J Chem Thermodyn. 1997;29:623–7.
Malyshev VM, Milner GA, Sorkin EL, Shibakin VF. Automatic low-temperature calorimeter. Pribory i Tekhnika Eksperimenta. 1985;6:195–7.
Yagfarov MSh. New method of measuring the heat capacity and heat effects. Zh Fiz Khimii. 1969;43:1620–5.
Kabo AG, Diky VV. Details of calibration of a scanning calorimeter of the triple heat bridge type. Thermochim Acta. 2000;347:79–84.
Maczka M, Ko J-H, Wlosewicz D, Tomaszewski PE, Kojima S, Hanuza J, et al. Heat capacity and dielectric studies of ferroelectric superionic conductor RbNbWO6. Solid State Ionics. 2004;167:309–15.
Lebedev BV. Application of precise calorimetry in study of polymers and polymerization processes. Thermochim Acta. 1997;297:143–9.
Chase MW Jr. NIST-JANAF themochemical tables, 4th ed. J Phys Chem Ref Data Monogr. 1998;9:1951 (database http://webbook.nist.gov/chemistry/).
Cox JD, Wagman DD, Medvedev VA. Codata key values for thermodynamics. New York; 1984 (database http://webbook.nist.gov/chemistry/).
Acknowledgments
The work was performed with the financial support of NNSU’s innovation educational program within the National project “Education”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knyazev, A., Mączka, M., Kuznetsova, N. et al. Thermodynamic properties of rubidium niobium tungsten oxide. J Therm Anal Calorim 98, 843–848 (2009). https://doi.org/10.1007/s10973-009-0112-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0112-6