Abstract
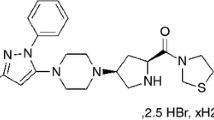
The thermal properties of verapamil hydrochloride (VRP) and its physical association as binary mixtures with some common excipients were evaluated. Thermogravimetry (TG) was used to determine the thermal mass loss, as well as to study the kinetics of VRP thermal decomposition, using the Flynn-Wall-Ozawa model. Based on their frequent use in pharmacy, five different excipients (microcrystalline cellulose, magnesium stearate, hydroxypropyl methylcellulose, polyvinylpyrrolidone and talc) were blended with VRP. Samples were prepared by mixing the analyte and excipients in a proportion of 1:1 (m/m). DSC curves for pure VRP presented an endothermic event at 143 ± 2 °C (ΔHmelt = 132 ± 4 J g−1), which corresponds to the melting (literature Tm = 143.7 °C, ΔHmelt = 130.6 J g−1). Comparisons among the observed results for each compound and their binary physical mixtures presented no relevant changes. This suggests no interaction between the drug and excipient.





Similar content being viewed by others
References
Balestrieri F, Magri AD, Magri AL, Marini D, Sacchini A. Application of differential scanning calorimetry to the study of drug-excipient compatibility. Thermochim Acta. 1996;285:337–45.
Cotton ML, Wu DW, Vadas EB. Drug excipient interaction study of enalapril maleate using thermal-analysis and scanning electron-microscopy. Int J Pharm. 1987;40:129–42.
Li Wan Po A, Mroso PV. Drug–drug incompatibility in the solid state: kinetic interpretation, modelling and prediction. Int J Pharm. 1984;18:287–98.
Mroso PV, Li Wan Po A, Irwin WJ. Solid-state stability of aspirin in the presence of excipients—kinetic interpretation, modeling, and prediction. J Pharm Sci. 1982;71:1096–101.
Botha SA, Lotter AP. Compatibility study between atenolol and tablet excipients using differential scanning calorimetry. Drug Dev Ind Pharm. 1990;16:1945–54.
Vantonder EC, Lotter AP, Botha SA. Compatibility study between doxylamine succinate with other drugs and excipients using differential scanning calorimetry. Drug Dev Ind Pharm. 1990;16:2125–33.
Donauer N, Lönbenberg R. A mini review of scientific and pharmacopeial requirements for the disintegration test. Int J Pharm. 2007;345:2–8.
Schomerus M, Spiegelhaider B, Stieren B, Eichelbaum M. Physiological disposition of verapamil in man. Cardiovasc Res. 1976;10:605–12.
Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–66.
Flynn JH, Wall LA. General treatment of thermogravimetry of polymers. J Res Natl Bur Stand A: Phys Chem. 1966;70:487–523.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17:407–33.
Flynn JH, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci B: Polym Lett. 1966;4:323–8.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Rustichelli C, Gamberini MC, Ferioli V, Gamberini G. Properties of the racemic species of verapamil hydrochloride and gallopamil hydrochloride. Int J Pharm. 1999;178:111–20.
Abbas D, Kaloustian J, Orneto C, Piccerelle P, Portugal H, Nicolay A. DSC and physico-chemical properties of a substituted pyridoquinoline and its interaction study with excipients. J Therm Anal Calorim. 2008;93:353–60.
American standard test method for oxidation onset temperature of hydrocarbons by differential scanning calorimetry, vol. 14.02. PA: ASTM International E2009-08; 2008.
Guinesi LS, Ribeiro CA, Crespi MS, Santos AF, Capela MV. Titanium(IV)–EDTA complex. J Therm Anal Calorim. 2006;85:301–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nunes, R.S., Semaan, F.S., Riga, A.T. et al. Thermal behavior of verapamil hydrochloride and its association with excipients. J Therm Anal Calorim 97, 349–353 (2009). https://doi.org/10.1007/s10973-009-0072-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0072-x