Abstract
Many concerns over unsafe or unknown properties of multi-walled carbon nanotubes (MWNTs) have been raised. The thermal characteristics regarding stability would represent potential hazards during the production or utilization stage and could be determined by calorimetric tests for various thermokinetic parameters. Differential scanning calorimetry (DSC) was employed to evaluate the thermokinetic parameters for MWNTs at various compositions.
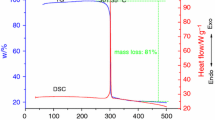
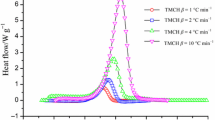
Thermoanalytical curves showed that the average heat of decomposition (ΔH d) of the MWNTs samples in a manufacturing process was about 31,723 J g−1, by identifying them as an inherently hazardous material. In this study, significant thermal analysis appeared in the presence of sulfuric acid (H2SO4). From the DSC experiments, the purification process of MWNTs could induce an unexpected reaction in the condition of batch addition with reactants of H2SO4. The results can be applied for designing emergency relief system and emergency rescue strategies during a perturbed situation or accident.
Similar content being viewed by others
References
S. Iijima, Nature, 354 (1991) 56.
K. A. Dean, T. P. Burgin and B. R. Chalamala, Appl. Phys. Lett., 79 (2001) 121.
H. Yu, C. Yu, T. Xi, L. Luo, J. Ning and C. Xiang, J. Therm. Anal. Cal., 82 (2005) 97.
T. Kashiwagi, E. Grulke, J. Hilding, K. Groth, R. Harris, K. Butler, J. Shields, S. Kharchenko and J. Douglas, Polymer, 45 (2004) 4227.
M. S. Dresselhaus, G. Dresselhaus and R. Satio, Carbon, 33 (1995) 883.
T. W. Ebbesen, Annu. Rev. Mater. Sci., 24 (1994) 235.
W. A. de Heer, Science, 270 (1995) 1179.
D. K. Pritchard, Literature Review-Explosion Hazards Associated with Nanopowders, HSL/2004/12,, 2004.
J. Jin, M. Song and F. Pan, Thermochim. Acta, 2007, DOI:10. 1016/j. tca. 02. 003.
S. Chervin and G. T. Bodman, Process Saf. Prog., 16 (1997) 94.
Y. W. Wang, C. M. Shu, Y. S. Duh and C. S. Kao, Ind. Eng. Chem. Res., 40 (2001) 1125.
C. M. Shu and Y. J. Yang, Thermochim. Acta, 392 (2002) 257.
M. K. Seo, J. R. Lee and S. J. Park, Mater. Sci. Eng., A, 404 (2005) 79.
Mettler Toledo, STARe Thermal Analysis, Sweden 2005.
W. Jang, Chemistry Department, Drexel University.
Mettler Toledo Company, TA8000 Operation Instructions, Switzerland 2003.
Y. S. Duh, C. S. Kao, C. Lee, C. C. Hsu and S. W. Yu, The 2nd International Conference and Exhibition on Loss Prevention in the Oil, Chemical and Process Industries, Singapore 1995.
Y. S. Duh, C. S. Kao, C. Lee, M. L. Chen and S. W. Yu, International Symposium on Runaway Reactions and Pressure Relief Design, 237, Boston, MA, USA 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, C.W., Chou, Y.C., Tseng, J.M. et al. Thermal hazard evaluation of carbon nanotubes with sulfuric acid by DSC. J Therm Anal Calorim 95, 639–643 (2009). https://doi.org/10.1007/s10973-008-9163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9163-3