Abstract
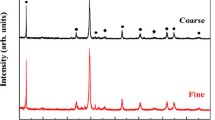
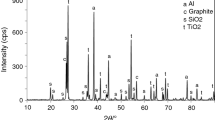
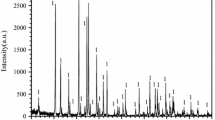
The oxidation kinetics of Zr-disilicide (ZrSi2) powders up to temperatures of 1550°C were studied in flowing air using non-isothermal and isothermal thermogravimetric (TG) analysis. During the oxidation process two main thermal events were detected. The first stage of the oxidation reaction leads to the formation of elemental silicon as an intermediate reaction product. Upon further temperature increase the newly formed silicon is oxidized. Completely oxidized ZrSi2 samples consist of ZrSiO4, amorphous and crystalline SiO2 as well as some residual ZrO2. The experimental TG data were analysed with a model-fitting kinetic method. The gas-solid reaction is complex and can best be fitted with a multi-step reaction scheme consisting of branching reactions based on 3D diffusion mechanisms and a fractal order reaction.
Similar content being viewed by others
References
H. M. Ondik and H. F. McMurdie, Phase Diagrams for Zirconium + Zirconia Systems, The American Ceramic Society 1998, p. 423.
H. Okamato, Bull. Alloy Phase Diagrams, 11 (1990) 513.
V. D. Hennige, J. Haußelt, H.-J. Ritzhaupt-Kleissl and T. Windmann, J. Eur. Ceram. Soc., 19 (1999) 2901.
N. Claussen, T. Le and S. Wu, J. Eur. Ceram. Soc., 5 (1989) 29.
O. Hönigschmid, Monatsh. Chem., 27 (1906) 1069.
V. A. Lavrenko, V. Zh. Shemet and A. V. Goncharuk, Thermochim. Acta, 93 (1985) 501.
J. Opfermann, Netzsch Thermokinetics 2, Version 2004.05, Netzsch Gerätebau GmbH.
H. Geßwein, J. R. Binder, H.-J. Ritzhaupt-Kleissl and J. Haußelt, J. Eur. Ceram. Soc., 26 (2006) 697.
J. Opfermann, J. Therm. Anal. Cal., 60 (2000) 641.
C. Wagner, J. Electrochem. Soc., 99 (1952) 368.
C. Wagner, J. Electrochem. Soc., 103 (1956) 627.
C. Wagner, Z. Electrochemie, 63 (1959) 773.
H. Geßwein and J. R. Binder, Thermochim. Acta, 444 (2006) 6.
J. P. Sanders and P. K. Gallagher, J. Therm. Anal. Cal., 72 (2003) 777.
B. Serin and T. Ellickson, J. Chem. Phys., 9 (1941) 742.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geßwein, H., Pfrengle, A., Binder, J.R. et al. Kinetic model of the oxidation of ZrSi2 powders. J Therm Anal Calorim 91, 517–523 (2008). https://doi.org/10.1007/s10973-007-8461-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-007-8461-5