Abstract
There is an increasing concern with the environmental problems associated with the increasing CO2, NOx and SOx emissions resulting from the rising use of fossil fuels. Renewable energy, mainly biomass, can contribute to reduce the fossil fuels consumption. Biomass is a renewable resource with a widespread world distribution. Pistachio is available in large quantities in Gaziantep region in Turkey. Pistachio shell has a good energy potential for exploitation through pyrolysis and gasification.
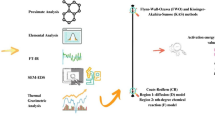
This study deals with the thermal degradation characteristics of in different particle sizes pistachio shell and its kinetics. Thermal degradation analysis have been done by using a thermogravimetric analyzer from room temperature to 800°C in N2 atmosphere at different heating rates (5, 10, 15 and 20°C min−1). TG and DTG curves exhibited two distinct degradation zones. Kinetic parameters were calculated by using Coats-Redfern method and model-free isoconversional Flynn-Wall-Ozawa (FWO) kinetic method.
Similar content being viewed by others

References
S. Yaman, Energ. Convers. Manage., 45 (2004) 651.
H. H. Acma, J. Anal. Appl. Pyrolysis, 75 (2005) 211.
V. Mangut, E. Sabio, J. Ganan, J. F. Gonzalez, A. Ramiro, C. M. Gonzalez, S. Roman and A. Al-Kassir, Fuel. Process. Technol., 87 (2006) 109.
T. Fisher, M. Hajaligol, B. Waymack and D. Kellog, J. Anal. Appl. Pyrolysis, 62 (2002) 331.
M. Pietro and C. Paola, Thermochim. Acta, 413 (2004) 209.
J. Peuravuori, N. Paaso and K. Pijlaha, Thermochim. Acta, 361 (1999) 181.
M. V. Kök and M. R. Pamir, J. Anal. Appl. Pyrolysis, 35 (1995) 145.
G. Steiner, J. Rath, M. G. Wolfinger and G. Staudinger, Thermochim. Acta, 398 (2003) 59.
M. V. Kök, G. Pokol, C. Keskin, J. Madarász and S. Bagci, J. Therm. Anal. Cal., 76 (2004) 247.
M. V. Kök, J. Therm. Anal. Cal., 64 (2001) 1319.
J. M. Nazzal, J. Therm. Anal. Cal., 65 (2001) 847.
S. Vyazovkin and C. A. Wight, Thermochim. Acta, 340 (1999) 53.
R. Lopez-Fonseca, I. Landa, M. A. Guiterrez-Ortiz and J. R. Gonzalez-Velasco, J. Therm. Anal. Cal., 80 (2005) 65.
M. A. Olivella and F. X. C. de la Heras, Thermochim. Acta, 385 (2002) 171.
Y. Tonbul and K. Yurdakoc, Turk. J. Chem., 25 (2001) 333.
M. V. Kök, Energ. Source, 25 (2003) 1007.
M. Z. Duz, Y. Tonbul, A. Baysal, O. Akba, A. Saydut and C. Hamamci, J. Therm. Anal. Cal., 81 (2005) 395.
Y. Tonbul, A. Saydut and C. Hamamci, Oil Shale, 23 (2006) 286.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tonbul, Y. Pyrolysis of pistachio shell as a biomass. J Therm Anal Calorim 91, 641–647 (2008). https://doi.org/10.1007/s10973-007-8428-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-007-8428-6