Abstract
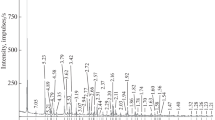
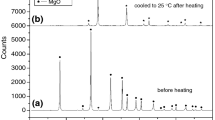
Emanation thermal analysis (ETA) was used to characterize the thermal reactivity of amorphous brannerite mineral of general formula U1–xTi2+xO6 (locality El Cabril, near Cordoba, Spain). It was demonstrated that on sample heating up to 880°C microstructure changes taking place in the sample were accompanied by the formation of new radon diffusion paths, followed by their closing up during the final transformation of amorphous to crystalline brannerite in the range 900–1020 °C. Relative changes in structure irregularities that served as radon diffusion paths during heating and subsequent cooling of the sample to temperatures of 300, 550, 750, 880, 1020 and 1130°C, respectively, were determined from the ETA results. Mass losses in temperature ranges of 230–315, 570–760 and 840–1040°C were observed by thermogravimetry. Mass spectrometry indicated the release of CO2 mainly due to the decomposition of minor carbon amount in the brannerite mineral sample.
Similar content being viewed by others
References
RO Ifill WC Cooper AH Clark (1996) CIM Bull. 89 93
JT Szymanski JD Scott (1982) Can. Mineral. 20 271 Occurrence Handle1:CAS:528:DyaL38XltFals7w%3D
GR Lumpkin SHF Leung M Colella (2000) Mater. Res. Soc. Symp. Proc. 608 359 Occurrence Handle1:CAS:528:DC%2BD3cXnslajtrg%3D
A. E. Ringwood, S. E. Kesson, K. D. Reeve, D. M. Levins and E. J. Ramm, in: W. Lutze, R. C. Ewing (Eds), Radioactive Waste Forms for the Future, 1988, p. 233.
JE Patchett EW Nuffield (1960) Can. Mineral. 6 483 Occurrence Handle1:CAS:528:DyaF3MXht1emur4%3D
E. R. Vance, M. W. A. Stewart, R. A. Day, K. P. Hart, M. J. Hambley and A. Brownscombe, ‘Pyrochlore-rich Titanate Ceramics for Incorporation of Plutonium, Uranium and Process Chemicals’, ANSTO report (1997).
ER Vance JN Watson ML Carter RA Day GR Lumpkin KP Hart Y Zhang PJ McGlinn MWA Stewart DJ Cassidy et al. (2000) Environmental Issues and Waste Management Technologies V American Ceramic Society USA 561
Y Zhang GR Lumpkin H Li MG Blackford M Colella ML Carter ER Vance (2006) J. Nucl. Mater. 350 293 Occurrence Handle10.1016/j.jnucmat.2006.01.012 Occurrence Handle1:CAS:528:DC%2BD28Xjslygt7s%3D
V Balek J Tölgyessy et al. (1984) Emanation thermal analysis and other radiometric emanation methods, in Wilson and Wilson’s Comprehensive Analytical Chemistry, Part XIIC Elsevier Science Publishers Amsterdam 304
V Balek ME Brown et al. (1998) Less common techniques, in: Handbook on Thermal Analysis and Calorimetry, Vol. 1 Chapter 9 Elsevier Science B.V. Amsterdam 445
JF Ziegler JP Biersack U Littmark et al. (1985) The Stopping and Range of Ions in Solids Pergamon Press New York
V Balek M Beneš Z Málek G Matuschek A Kettrup S Yariv (2006) J. Therm. Anal. Cal. 83 617 Occurrence Handle10.1007/s10973-005-7424-y Occurrence Handle1:CAS:528:DC%2BD28Xjt1Kgt78%3D
NK Labhsetwar V Balek S Rayalu T Terasaka A Yamazaki J Šubrt H Haneda T Mitsuhashi (2005) J. Therm. Anal. Cal. 80 67 Occurrence Handle10.1007/s10973-005-0712-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balek, V., Vance, E.R., Zeleňák, V. et al. Use of emanation thermal analysis to characterize thermal reactivity of brannerite mineral. J Therm Anal Calorim 88, 93–98 (2007). https://doi.org/10.1007/s10973-006-8094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-8094-0