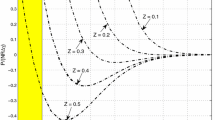
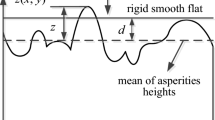
Abstract
Jäntti introduced a method to calculate the final value of the adsorbed mass already in a very early stage of adsorption measurements. His method was restricted to gravimetric measurements and to adsorptions, which satisfy the simplest molecular model. One of the advantages of his method was that when more complicated molecular models were necessary, the curve resulting from Jäntti`s calculations showed discrepancies from the curve predicted for the simple situation. We used such deviations to evaluate the parameters of the models necessary for the explanation of the measurements. One of the examples we discussed concerned the influence of the occurrence of two parallel and simultaneous adsorptions.
In the present paper we discuss the application of these results to adsorption onto a surface where roughness could be expected to play a part. If we consider a rough surface as the sum of two extra surfaces we can apply Jäntti’s method by using our former results of parallel adsorption. We characterise roughness by two parameters which we evaluated with Jäntti’s method. We emphasise that the existence of roughness is not demonstrated by Jäntti’s method, but that the method is useful for the evaluation of parameters introduced by other arguments or from other sources.
Similar content being viewed by others
References
O Jäntti J Junttila E Yrjänheikki (1970) Suomen Kemistilehti, A 43 214
O Jäntti J Junttila E Yrjänheikki et al. (1972) Progress in Vacuum Microbalance Techniques Heyden London 345
O Jäntti E Robens (1981) Thermochim. Acta 51 67 Occurrence Handle10.1016/0040-6031(81)87051-7
CH Massen JA Poulis E Robens (2000) Adsorption 6 229 Occurrence Handle1:CAS:528:DC%2BD3cXnvFWgtb8%3D Occurrence Handle10.1023/A:1008989227791
J. A. Poulis, J. Adolphs, E. Robens and C. H. Massen, in Theoretical and Experimental Studies of Interfacial Phenomena and their Technological Applications: Book of Abstracts, VIII Ukrainian-Polish Symposium, September 19–24. 2004, Sergijiwka-Odesa, Ukraine, Y. Tarasevich, R. Leboda, B. Kats and E. Aksenenko, Eds, SCSEIO, Odessa 2004, pp. 258–263.
JA Poulis CH Massen E Robens (2002) J. Therm. Anal. Cal. 68 719 Occurrence Handle1:CAS:528:DC%2BD38XkvFegtrY%3D Occurrence Handle10.1023/A:1016028911495
JA Poulis CH Massen E Robens (2003) J. Therm. Anal. Cal. 71 61 Occurrence Handle1:CAS:528:DC%2BD3sXptFyisA%3D%3D Occurrence Handle10.1023/A:1022201930553
JA Poulis et al. (2002) Characterization of Porous Solids VI. Elsevier Amsterdam
JA Poulis CH Massen E Robens G Reichenauer (2004) Z. Phys. Chem. 218 245 Occurrence Handle1:CAS:528:DC%2BD2cXitFKitrY%3D
JA Poulis CH Massen E Robens G Reichenauer (2004) J. Therm. Anal. Cal. 76 583 Occurrence Handle1:CAS:528:DC%2BD2cXktVWhtb8%3D Occurrence Handle10.1023/B:JTAN.0000028037.48986.63
JA Poulis C H.Massen E Robens KK Unger et al. (1999) Characterisation of Porous Solids Elsevier Amsterdam 151
JA Poulis G Reichenauer CH Massen E Robens (2002) Z. Phys. Chem. 216 1123 Occurrence Handle1:CAS:528:DC%2BD38XnvFSrsL8%3D
JA Poulis E Robens CH Massen G Reichenauer (2004) J. Therm. Anal. Cal. 76 579 Occurrence Handle1:CAS:528:DC%2BD2cXktVWhtb4%3D Occurrence Handle10.1023/B:JTAN.0000028036.60418.4a
E Robens CH Massen JA Poulis P Staszczuk (1999) Adsorpt. Sci. Technol. 17 801 Occurrence Handle1:CAS:528:DC%2BD3cXhvV2qsbg%3D
E Robens JA Poulis CH Massen (2000) J. Therm. Anal. Cal. 62 429 Occurrence Handle1:CAS:528:DC%2BD3MXptlel Occurrence Handle10.1023/A:1010190131304
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poulis, J.A., Robens, E., Massen, C.H. et al. Application of Jäntti’s method for the explanation of adsorption onto rough surfaces. J Therm Anal Calorim 86, 39–42 (2006). https://doi.org/10.1007/s10973-006-7591-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7591-5